Antibody Scaffolds
Scientific Digital Marketing, Synthetic Biology, Nucleic Acid Therapeutics and Antibody Engineering, Biotech Writer
An antibody scaffold is a minimalistic protein framework derived from natural antibodies (immunoglobulins) designed to retain the ability to specifically recognize and bind target molecules (antigens). Unlike full-length antibodies which are large, multi-domain glycoproteins (~150 kDa) comprising two heavy chains and two light chains antibody scaffolds typically consist of smaller protein domains or engineered fragments, retaining primarily the antigen-binding sites and their adjacent structural motifs necessary for specificity.
Antibody scaffolds are engineered protein frameworks derived from the antigen-binding regions of natural antibodies, designed to retain the ability to specifically recognize and bind target molecules (antigens) while offering significant advantages over traditional full-length antibodies. Unlike conventional antibodies, which are large, multi-domain glycoproteins (~150 kDa) composed of two heavy and two light chains, antibody scaffolds are typically smaller, ranging from 10 to 50 kDa, and consist of simplified structural domains. These scaffolds retain the critical antigen-binding loops, known as Complementarity-Determining Regions (CDRs), which are responsible for specificity and affinity, while eliminating non-essential regions that contribute to size and complexity. This minimalist design not only enhances their functional versatility but also improves their physicochemical properties, making them ideal for a wide range of biomedical applications.
The structural simplicity of antibody scaffolds, such as Fab fragments, single-chain variable fragments (scFvs), and nanobodies, allows for deeper tissue penetration, rapid biodistribution, and efficient renal clearance. These properties are particularly advantageous in therapeutic contexts, where smaller scaffolds can access epitopes that are hidden or structurally constrained, such as those within dense tumor tissues or intracellular compartments. Additionally, their compact size and modular architecture facilitate genetic engineering, enabling researchers to tailor scaffolds for specific applications through techniques like site-directed mutagenesis, directed evolution, and computational design. This high degree of customization has led to the development of scaffolds with enhanced stability, reduced immunogenicity, and improved binding affinity, making them suitable for use in harsh environments, such as extreme pH or high temperatures, and for repeated freeze-thaw cycles in diagnostic assays.
Beyond their structural and functional advantages, antibody scaffolds have opened new frontiers in biomedicine. They are being employed as therapeutic agents in oncology, autoimmune diseases, and infectious diseases, where their ability to precisely target disease-causing molecules has led to breakthroughs in treatment efficacy and safety. In diagnostics, their small size and rapid clearance make them ideal for molecular imaging and biosensing applications, providing high-contrast images and sensitive detection of biomarkers. Furthermore, their engineering flexibility allows for conjugation with drugs, toxins, nanoparticles, and nucleic acids, enabling targeted drug delivery systems that minimize off-target effects and maximize therapeutic impact. As the field continues to evolve, emerging trends such as multispecific and multivalent scaffolds, intracellular targeting, AI-driven design, and advanced manufacturing techniques are pushing the boundaries of what these scaffolds can achieve, paving the way for transformative advances in precision medicine and biotechnology.
This article explores the fundamental principles, engineering strategies, and cutting-edge innovations in antibody scaffold technology, highlighting their potential to revolutionize healthcare. From their structural basis and technical advantages to their applications in therapeutics, diagnostics, and drug delivery, we delve into the science behind these remarkable molecules and their role in shaping the future of biomedicine.
Structural Components:
The binding specificity of antibodies originates from Complementarity-Determining Regions (CDRs) within the Variable (V) domains of the antibody heavy (VH) and light (VL) chains. These CDR loops directly interact with antigens and determine specificity and affinity. The scaffold itself maintains a stable structural backbone to position these loops optimally for antigen recognition.
Typical antibody scaffolds are derived by:
Isolating Fab (fragment antigen-binding) regions (~50 kDa), consisting of VH, CH1, VL, and CL domains.
Creating single-chain variable fragments (scFvs) (~25-30 kDa), linking VH and VL domains via flexible peptide linkers (commonly Glycine-Serine-rich sequences).
Using single-domain scaffolds (VHH or Nanobodies, ~12-15 kDa) derived from camelid antibodies lacking light chains, which naturally have enhanced stability and solubility.
Engineering and Customization:
Antibody scaffolds are particularly attractive due to their high modularity and ease of genetic engineering. Techniques commonly applied include:
Site-directed mutagenesis to modify amino acids within CDRs for affinity maturation or specificity alteration.
Directed evolution (phage display, yeast display, ribosome display) to screen vast libraries of variants, identifying optimal scaffolds with high specificity and affinity.
Computational design methods to rationally optimize binding characteristics, stability, aggregation resistance, and pharmacokinetic properties.
Advantages Over Conventional Antibodies:
Reduced Size: Improved tissue penetration, enhanced biodistribution, and accelerated clearance rates from non-target tissues.
Increased Stability: Engineered antibody scaffolds, such as nanobodies or engineered Fab fragments, often exhibit higher thermal, chemical, and proteolytic stability compared to full-length antibodies.
Simplified Production: Recombinant antibody scaffolds can be efficiently expressed in microbial systems (E. coli, yeast), which reduces manufacturing costs, time, and complexity.
Engineering Flexibility: Ease of conjugation with imaging agents, drugs, toxins, nanoparticles, or polymers, enabling versatile therapeutic and diagnostic applications.
Examples of Engineered Antibody Scaffolds:
Nanobodies (VHH): Derived from camelids, these single-domain antibodies maintain the VH domain with unique structural features (e.g., extended CDR3 loops), offering exceptional stability and specificity.
DARPins (Designed Ankyrin Repeat Proteins): Non-immunoglobulin scaffolds mimicking antibody binding properties, engineered to exhibit high-affinity protein-protein interactions.
Affibodies: Engineered from protein-A domains, designed for high-affinity binding, robustness, and ease of manufacturing.
Applications:
Therapeutics: Targeted therapies for oncology (e.g., CAR-T cell targeting domains), autoimmune conditions, viral infections, and chronic diseases.
Diagnostics & Imaging: Radiolabeled or fluorescently labeled scaffolds for molecular imaging or diagnostic assays due to superior tissue penetration and fast clearance.
Drug Delivery Systems: Conjugated antibody scaffolds serving as targeting moieties for precision delivery of payloads (e.g., toxins, small molecules, nucleic acids).
Antibody scaffolds leverage the fundamental structural elements of antibodies particularly the antigen-binding loops in a minimized, engineered format. Their molecular simplicity, ease of customization, high specificity, and enhanced physicochemical properties have made them versatile tools in biomedicine, therapeutics, diagnostics, and biotechnology.
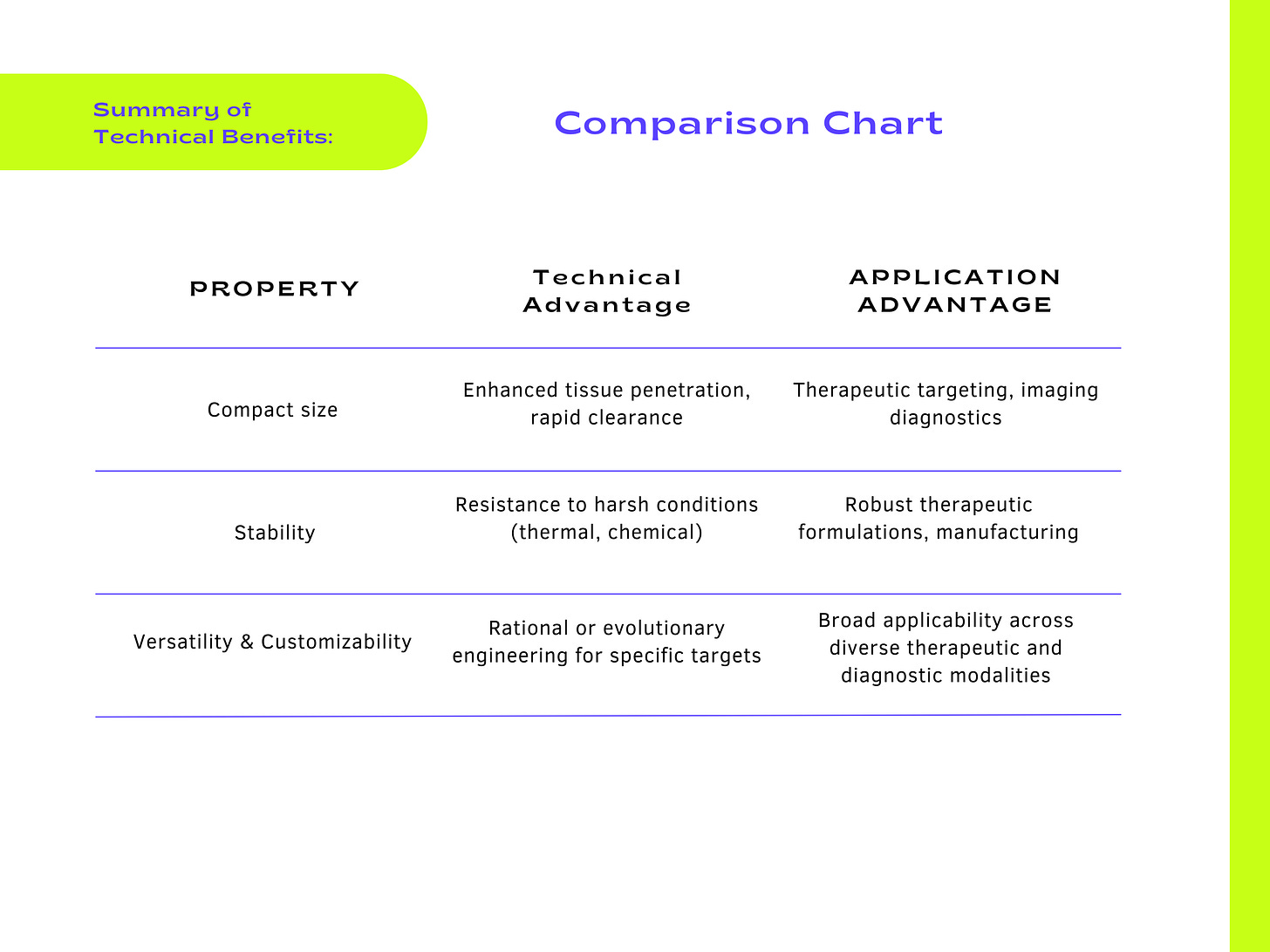
Enhanced Tissue Penetration: Smaller molecular weight and reduced hydrodynamic radius enable rapid and deeper penetration into dense tissues or tumors. Improved diffusion coefficients result in more homogeneous distribution within target tissues, overcoming diffusion limitations observed with conventional antibodies.
Improved Pharmacokinetics and Bioavailability: Rapid clearance from systemic circulation (short half-life) decreases potential systemic toxicity and increases the therapeutic window, especially for radiolabeled imaging agents. Efficient renal clearance due to their size (typically <60 kDa), reducing off-target toxicity and enhancing safety profiles.
Facilitation of Cellular Access: Small scaffolds may access epitopes hidden within protein complexes or membrane-proximal epitopes inaccessible to larger antibodies. Ability to target cryptic epitopes or structurally constrained binding sites due to their minimized steric hindrance.
2. High Stability (Thermal, Chemical, and Proteolytic Stability)
Structural Engineering for Stability: Mutation of hydrophobic amino acids to hydrophilic residues to improve aqueous solubility and reduce aggregation propensity. Stabilizing domain folding by introducing disulfide bonds or salt bridges to lock protein conformations into stable states.
Resistance to Denaturation: High thermal stability (e.g., stable at temperatures above 60–80°C), beneficial for diagnostic applications requiring heat stability or repeated freeze-thaw cycles. Chemically resistant, tolerating harsh conditions (pH extremes, chaotropic agents, detergents), suitable for industrial processes, diagnostic assays, and pharmaceutical formulations.
Proteolytic Resistance: Engineering for reduced susceptibility to proteolytic enzymes through targeted amino acid substitutions that remove cleavage-prone residues (arginine, lysine, proline). Utilization of compact structural domains with minimal exposed flexible regions, making them inherently less prone to enzymatic digestion or degradation.
3. Versatility and Customizable Binding Sites
Complementarity-Determining Region (CDR) Engineering: Directed mutagenesis or randomized library screening (e.g., via phage display or yeast display) to precisely alter amino acids in antigen-binding loops. Computational design to predict and refine affinity, specificity, and selectivity against complex targets (proteins, peptides, nucleic acids, carbohydrates, small molecules).
Modular Framework Designs: Scaffold frameworks engineered with defined positions that tolerate amino acid diversity without losing structural integrity. Ability to rapidly exchange or alter binding loops, enabling quick generation of customized scaffolds against novel or challenging targets.
Versatile Targeting Applications: Ability to engineer scaffolds that recognize epitopes that are inaccessible or weakly immunogenic to traditional antibodies, including active sites on enzymes, conformational epitopes, intracellular targets, or membrane-proximal regions. Facilitating multi-specific targeting (e.g., bi-specific or tri-specific scaffolds), achieved by combining distinct scaffold domains or coupling scaffolds to complementary molecular functionalities.
Fab Fragments (Fragment Antigen-Binding)
Structural Features:
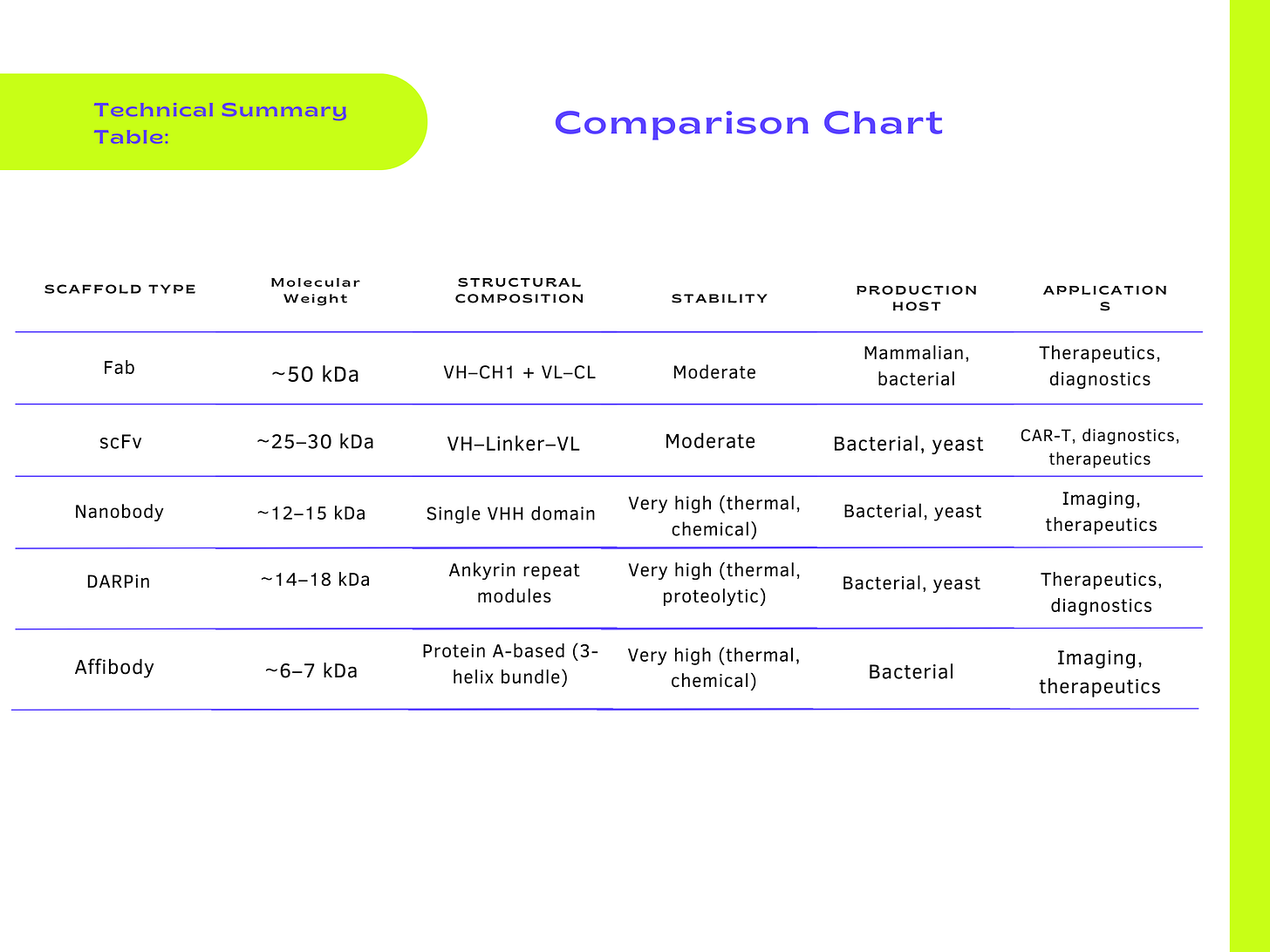
Composition: Fab fragments (~50 kDa) are composed of two distinct polypeptide chains: Heavy-chain variable (VH) and constant (CH1) domains. Light-chain variable (VL) and constant (CL) domains.
Linkage: Connected via disulfide bonds at the CH1-CL interface.
Antigen Binding: Includes full complementarity-determining regions (CDRs) in VH and VL, maintaining robust antigen specificity and affinity comparable to full antibodies.
Technical Advantages:
Retention of original binding affinity/specificity from parent antibodies.
Reduced immunogenicity due to removal of Fc region.
Moderate size balancing stability, affinity, and tissue penetration.
Applications:
Clinical therapeutics (e.g., Ranibizumab, Certolizumab pegol).
Diagnostic imaging and targeted drug delivery.
2. scFv (Single-Chain Variable Fragment)
Structural Features:
Composition: Single polypeptide chain (~25–30 kDa), consisting of VH and VL domains fused via a flexible peptide linker (commonly a Glycine–Serine linker, typically (Gly4Ser)3 or similar).
Linker Functionality: Maintains correct domain orientation to preserve antigen-binding conformation.
Technical Advantages:
High modularity and customization: Easily expressed in bacterial (E. coli) or yeast expression systems. Enables rapid combinatorial screening (phage display, yeast display).
Reduced Size: Improved tissue penetration and rapid renal clearance. Allows tandem fusions for multi-specific targeting.
Applications:
CAR-T cell therapies (antigen-targeting domains).
Recombinant immunotoxins and antibody-drug conjugates (ADC).
Diagnostics (biosensors, ELISA assays).
3. Nanobodies (Single-Domain Antibodies, VHH)
Composition: Single-domain antibodies (~12–15 kDa) derived from camelid heavy-chain-only antibodies (llamas, alpacas, camels).
Unique Structural Traits: Absence of a light chain; the antigen-binding capacity resides exclusively within a single VH domain (VHH). Extended hypervariable loop regions (particularly elongated CDR3 loops) facilitate binding into concave or cryptic epitopes.
Technical Advantages:
Exceptional Stability: High thermostability (stable above 70°C–90°C). Chemical resistance, including extreme pH, detergents, and proteolytic enzymes.
High Solubility and Low Aggregation: Engineered for minimal aggregation, ideal for therapeutic and diagnostic formulations.
Small Size: Rapid biodistribution, excellent tissue penetration, and renal clearance.
Applications:
Therapeutic agents (oncology, immunotherapy).
Diagnostics, molecular imaging (radiolabeled or fluorescent nanobodies).
Targeting intracellular or inaccessible epitopes.
4. DARPins (Designed Ankyrin Repeat Proteins)
Composition: Non-immunoglobulin scaffold (~14–18 kDa per repeat), composed of repetitive ankyrin repeat modules, typically containing stacked α-helices and β-turns forming a compact, stable core.
Customization: Specificity defined by amino acid substitutions within designated binding loops connecting ankyrin repeats.
Technical Advantages:
Exceptional Stability and Robustness: Resistant to thermal denaturation (up to 90°C or higher), proteolysis, and chemical stress. Ease of recombinant production in bacteria or yeast (high yield and purity).
High Binding Affinity and Specificity: Achievable picomolar to nanomolar affinities through library screening and directed evolution.
Structural Modularity: Easily engineered tandem arrays to create multi-specific or multi-valent binding molecules.
Applications:
Therapeutics targeting receptors or enzymes.
Biosensors, diagnostic assays, and imaging probes.
Delivery platforms for drug conjugation.
5. Affibodies (Protein A-derived Scaffold)
Composition: Small (~6–7 kDa) engineered scaffold protein derived from the B-domain of Staphylococcal Protein A.
Structural Basis: Three-helix bundle architecture, forming a stable, compact binding domain. Binding surfaces generated through amino acid diversity introduced primarily in helix regions.
Technical Advantages:
Exceptional Stability: High thermal stability (stable up to ~85–95°C). Resistance to harsh chemical environments.
Rapid Production: Expressed efficiently in E. coli, providing straightforward purification.
High Specificity and Affinity: Engineered affinities typically in low nanomolar to picomolar ranges, optimized via display technologies (e.g., phage, yeast).
Applications:
Molecular imaging (radiolabeled affibodies in PET imaging, such as HER2-targeting Affibody).
Tumor targeting and therapeutic payload delivery.
Biosensors and diagnostic platforms
Engineering Antibody Scaffolds
Antibody scaffold engineering involves a combination of rational design, molecular evolution, library construction, and high-throughput selection techniques to optimize and customize scaffolds for specific targets.
The process typically consists of several interconnected technical steps:
1. Scaffold Selection and Design
The first step involves choosing a suitable starting framework. Typically used frameworks include:
Fab fragments (VH+VL domains with CH1 and CL domains)
scFvs (single-chain variable fragments with VH-VL fusion)
Nanobodies (VHH domains) from camelids
Non-Ig-based scaffolds (DARPins, Affibodies)
Rational Scaffold Design:
Computational modeling (e.g., Rosetta, AlphaFold, molecular dynamics) is used to predict structural stability, identify mutable regions (CDRs), and design optimized interactions.
Stabilizing mutations (disulfide bridges, salt bridges, hydrophobic core modifications) are introduced to enhance thermal, chemical, or proteolytic stability.
Engineering Steps
1. Library Construction
Libraries represent diverse collections of scaffold variants with variations in key binding regions, typically generated through:
Random mutagenesis (e.g., error-prone PCR): Introduces random amino acid substitutions into scaffold CDR loops or binding interfaces.
Site-directed mutagenesis: Precise amino acid substitutions at defined positions known to influence antigen affinity, stability, or specificity.
Synthetic DNA library construction: Controlled, chemically synthesized oligonucleotides encoding randomized or semi-randomized amino acid diversity at specific positions (e.g., NNK codon strategy for amino acid diversity).
Scaffold Library Formats:
Phage Display: DNA encoding scaffold variants fused to phage coat proteins (e.g., pIII protein), displayed on bacteriophage surfaces.
Yeast Display: Scaffold variants displayed on yeast cell surfaces via fusion to surface proteins (e.g., Aga2), allowing quantitative affinity maturation and characterization.
Ribosome Display: Cell-free technique linking mRNA directly to scaffold protein variants, enabling extensive diversity and rapid selection.
High-throughput Selection and Screening Methods
Scaffold library displayed on bacteriophages exposed to immobilized or solution-phase antigens.
Specific binders enriched via multiple rounds of selection (biopanning).
Selected variants sequenced, cloned, and further characterized.
Technical considerations:
High diversity (>10^9 variants) achievable.
High throughput but limited quantitative affinity data.
Yeast Surface Display & Fluorescence-Activated Cell Sorting (FACS)
Scaffold variants displayed on yeast cell surfaces incubated with fluorescently labeled antigen.
Fluorescence-activated cell sorting (FACS) used for quantitative selection, enabling precise affinity measurement and optimization in real-time.
Technical advantages:
Quantitative, real-time assessment of binding kinetics and affinity.
Ideal for affinity maturation via iterative selection rounds.
Affinity Maturation and Stability Engineering
Iterative cycles of mutagenesis and selection (via yeast/phage display).
Focuses mutations primarily on CDR regions, specifically loops directly interacting with antigen surfaces.
Computational approaches (molecular docking, machine learning) guide rational mutation selection.
Stability Enhancement Techniques:
Computational protein redesign (Rosetta design, FoldX, AlphaFold): Predict stabilizing mutations (salt bridges, disulfide bonds).
Experimental screening methods: Thermostability assays (Differential Scanning Fluorimetry, Circular Dichroism). Aggregation propensity assessments (SEC-HPLC, SEC-MALS).
Conjugation and Functionalization
Incorporation of reactive amino acids (cysteines, unnatural amino acids) for conjugation to drugs, imaging agents, nanoparticles, or surfaces.
Genetic fusion with functional domains (enzyme domains, fluorescent proteins, cytokines) enhances therapeutic efficacy or diagnostics.
Technical methods:
Chemical conjugation (maleimide-thiol chemistry, click chemistry): Allows precise, site-specific payload attachment.
Genetic fusions: Fusion to fluorescent proteins (GFP variants) or enzymes (horseradish peroxidase, alkaline phosphatase) for diagnostic applications.
Target Validation and Biological Characterization
Surface Plasmon Resonance (SPR): Accurate kinetic analysis (association and dissociation rates), determining equilibrium dissociation constants (Kd values).
Flow Cytometry and Cell-Based Assays: Confirm specificity, binding affinity, and functional efficacy against cells expressing native target antigens.
In Vivo Studies: Animal models validate pharmacokinetics, tumor penetration, biodistribution, toxicity, and therapeutic efficacy.
Examples of Technical Applications:
Oncology: Engineered nanobody–drug conjugates targeting EGFR-positive tumors.
Autoimmune diseases: TNF-α-targeted Fab fragments engineered for minimal immunogenicity and optimal pharmacokinetics.
Infectious Diseases: Anti-SARS-CoV-2 nanobodies, optimized via yeast display for high-affinity neutralization and stability.
Summary of Technical Workflow:
1. Initial Scaffold Selection and Rational Design
2. Library Generation (mutagenesis, computational methods)
3. High-throughput screening (phage display, yeast display)
4. Affinity maturation (iterative cycles of display and selection)
5. Stability enhancement and conjugation optimization
6. Validation through kinetic assays (SPR), cell-based assays, and animal models
Therapeutic Agents
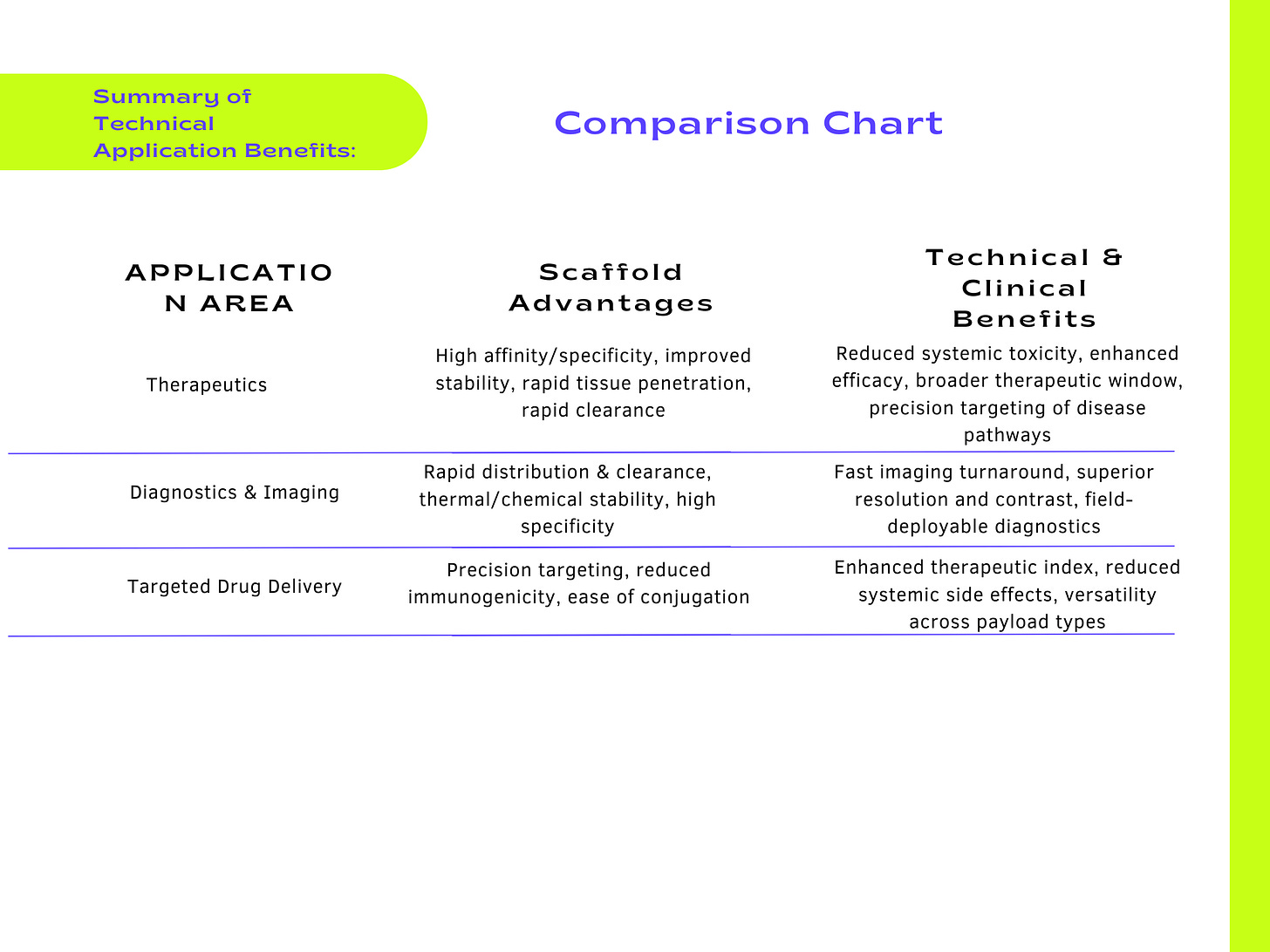
Antibody scaffolds have emerged as promising therapeutic agents due to their tailored specificity, enhanced stability, compact size, and adaptability to various targets. Specific therapeutic applications include:
Oncology Therapeutics
Mechanism: Engineered scaffolds specifically bind tumor-associated antigens (e.g., HER2, EGFR, CD20, VEGF), disrupting oncogenic signaling pathways. Direct tumor cell killing via immune-mediated mechanisms when fused to cytotoxic payloads or effector molecules.
Technical Advantages: Smaller scaffolds (nanobodies, affibodies, scFv) demonstrate improved tumor penetration, reaching poorly accessible tumor regions. Rapid clearance minimizes systemic toxicity.
Example: HER2-targeted Affibodies used for imaging and therapeutics in breast cancer. CD19-specific scFvs utilized in CAR-T cell therapies for leukemia and lymphoma.
Autoimmune Disease Therapeutics
Mechanism: Specific targeting of cytokines (e.g., TNF-α, IL-6, IL-17) or immune cell receptors (e.g., CD20, CD40) to modulate inflammatory responses.
Technical Advantages: Precision modulation of immune pathways reduces off-target effects. Rapid clearance reduces the risk of prolonged immune suppression.
Example: TNF-α-targeting Fab fragments (e.g., Certolizumab pegol) approved for rheumatoid arthritis and Crohn’s disease.
Infectious Disease Therapeutics
Mechanism: Binding and neutralization of microbial antigens, viral envelope proteins, or bacterial toxins.
Technical Advantages: Rapid neutralization due to fast biodistribution. Stability in harsh environments (e.g., gastrointestinal tract or respiratory mucosa), beneficial for prophylactic use.
Example: SARS-CoV-2-targeting Nanobodies and engineered scFvs designed to neutralize spike protein interactions.
2. Diagnostic Tools and Imaging Agents
Antibody scaffolds have transformed molecular diagnostics and imaging by virtue of their compact size, rapid clearance, and high specificity:
Diagnostic Assays and Biosensors
Mechanism: Highly specific antigen recognition facilitates sensitive detection and quantification in immunoassays (ELISA, lateral flow assays). Scaffolds immobilized on sensor surfaces for label-free real-time detection (SPR, Bio-Layer Interferometry).
Technical Advantages: Exceptional stability (thermal, chemical) enables robust diagnostic devices suitable for field use or point-of-care settings. Minimal non-specific interactions and low background signals, enhancing assay sensitivity.
Example: DARPin-based diagnostic sensors detecting biomarkers in serum or saliva. Nanobody-based lateral-flow assays for rapid infectious disease diagnostics (e.g., COVID-19 antigen tests).
Molecular Imaging Agents
Mechanism: Antibody scaffolds conjugated with radioisotopes (PET, SPECT), fluorescent dyes, or nanoparticles for targeted imaging of tumors or inflammatory lesions.
Technical Advantages: Fast tumor penetration and clearance from non-target tissues yield high-contrast images shortly after administration. Small size ensures rapid renal clearance, minimizing systemic exposure to radioactive isotopes or dyes.
Example: HER2-specific Affibodies labeled with ^68Ga for PET imaging in breast cancer. Fluorescent-labeled Nanobodies for intraoperative visualization of tumors or inflammation.
3. Targeting Agents for Drug Delivery Systems
Scaffold conjugated directly or indirectly to therapeutic payloads such as: Chemotherapeutics (small molecules, toxins) Radionuclides (radioimmunotherapy) siRNAs or nucleic acids Nanocarriers (liposomes, nanoparticles)
Technical Advantages:
Precision Targeting: High-affinity binding ensures targeted drug delivery and internalization, enhancing therapeutic index.
Reduced Immunogenicity and Toxicity: Removal of Fc domains or large immunogenic regions minimizes unintended immune responses. Rapid systemic clearance reduces off-target toxicity.
Examples:
Antibody-Drug Conjugates (ADC): Fab or scFv fragments linked to cytotoxic drugs, ensuring efficient tumor-specific delivery (e.g., scFv- or Fab-based ADCs).
Nanobody-based Drug Delivery: VHH domains conjugated with nanoparticles encapsulating chemotherapy drugs or siRNA to selectively target tumor cells or infected tissues.
Targeted Gene Delivery: Antibody scaffolds conjugated to liposomes or nanoparticles encapsulating nucleic acid therapeutics (siRNA, mRNA), enabling targeted gene therapy delivery.
The technical flexibility, stability, and binding precision of antibody scaffolds position them uniquely to innovate across therapeutics, diagnostics, imaging, and targeted drug delivery applications, driving advances in precision medicine and biomedical technology development.
Emerging Trends in Antibody Scaffold Engineering: Multispecificity, Intracellular Targeting, AI-Driven Design, and Advanced Manufacturing
Antibody scaffolds have revolutionized biomedicine by offering a versatile platform for engineering highly specific, stable, and customizable binding molecules. As the field advances, emerging trends are pushing the boundaries of what these scaffolds can achieve. This article explores the latest innovations in antibody scaffold engineering, focusing on multispecific and multivalent scaffolds, intracellular targeting, AI-driven design, advanced manufacturing techniques, and the challenges and solutions that accompany these advancements.
Multispecific and Multivalent Scaffolds
Multispecific and multivalent scaffolds represent a significant leap in therapeutic engineering, enabling simultaneous binding to multiple targets (multispecificity) or multiple copies of the same target (multivalency). This approach enhances therapeutic efficacy by facilitating synergistic interactions, such as immune cell recruitment, dual signaling pathway inhibition, or neutralization of multiple disease mediators. For example, in oncology, bispecific scaffolds can target both PD-1 and CTLA-4 immune checkpoints, amplifying anti-tumor immune responses. Similarly, in infectious diseases, bispecific nanobodies can neutralize multiple viral variants, such as different strains of the SARS-CoV-2 spike protein, providing broad-spectrum protection.
The engineering of multispecific scaffolds often involves the fusion of multiple scaffold domains, such as tandem nanobodies or bispecific single-chain variable fragments (scFvs). These domains are connected using flexible or rigid peptide linkers, such as glycine-serine-rich sequences, to ensure optimal spatial orientation and binding kinetics. Modular assembly techniques further streamline the process, allowing researchers to rapidly generate and test multispecific variants. This versatility has opened new avenues in therapeutic development, particularly in complex diseases where targeting multiple pathways or antigens is essential for efficacy.
Intracellular Targeting with Cell-Penetrating Scaffolds
While traditional antibody scaffolds excel at targeting extracellular proteins, many disease-causing molecules reside inside cells, making them inaccessible to conventional therapies. To address this limitation, researchers are engineering scaffolds capable of penetrating cell membranes and delivering therapeutic payloads to intracellular targets. This is achieved through fusion with cell-penetrating peptides (CPPs), such as the TAT peptide, which facilitate membrane translocation. Alternatively, pH-sensitive domains can be incorporated to exploit the acidic environment of endosomes, enabling endosomal escape and intracellular release.
Another strategy involves leveraging receptor-mediated endocytosis, where scaffolds are designed to bind cell surface receptors, such as transferrin receptors, which naturally internalize into cells. These approaches have proven particularly valuable in oncology, where intracellular oncoproteins like RAS and MYC, traditionally considered "undruggable," can now be targeted. Additionally, intracellular targeting enables the delivery of gene-editing tools, such as CRISPR-Cas9, or nucleic acids, such as siRNA and mRNA, for gene therapy applications. This innovation holds promise not only for cancer but also for infectious diseases, where intracellular viral replication machinery can be disrupted.
AI-Driven Scaffold Design and Optimization
The integration of artificial intelligence (AI) and machine learning (ML) into antibody scaffold engineering has accelerated the discovery and optimization of novel scaffolds. AI-driven tools, such as AlphaFold and Rosetta, predict scaffold structures and binding interactions with remarkable accuracy, enabling researchers to design scaffolds with enhanced affinity and stability. For instance, AI can predict the structure of nanobodies with extended CDR3 loops, allowing them to bind cryptic epitopes that are inaccessible to traditional antibodies.
Generative AI takes this a step further by creating entirely novel scaffold sequences with desired properties, such as high affinity for challenging targets like intrinsically disordered proteins. These computational tools also streamline the optimization process, predicting stabilizing mutations, such as disulfide bonds or salt bridges, to enhance scaffold robustness. By integrating data from high-throughput screening methods, such as phage display and yeast display, AI enables the rapid identification of optimal scaffolds, significantly reducing development timelines. This synergy between computational and experimental approaches is transforming scaffold engineering, making it faster, more efficient, and more precise.
Advanced Production and Manufacturing Techniques
As the demand for antibody scaffolds grows, so does the need for scalable and cost-effective production methods. Cell-free protein synthesis (CFPS) has emerged as a powerful tool for rapid prototyping and high-throughput screening. Unlike traditional cell-based systems, CFPS eliminates host cell constraints, such as toxicity and folding limitations, allowing for the efficient production of complex scaffolds. This method also facilitates the incorporation of non-natural amino acids, expanding the functional capabilities of scaffolds.
Challenges and Solutions in Antibody Scaffold Development
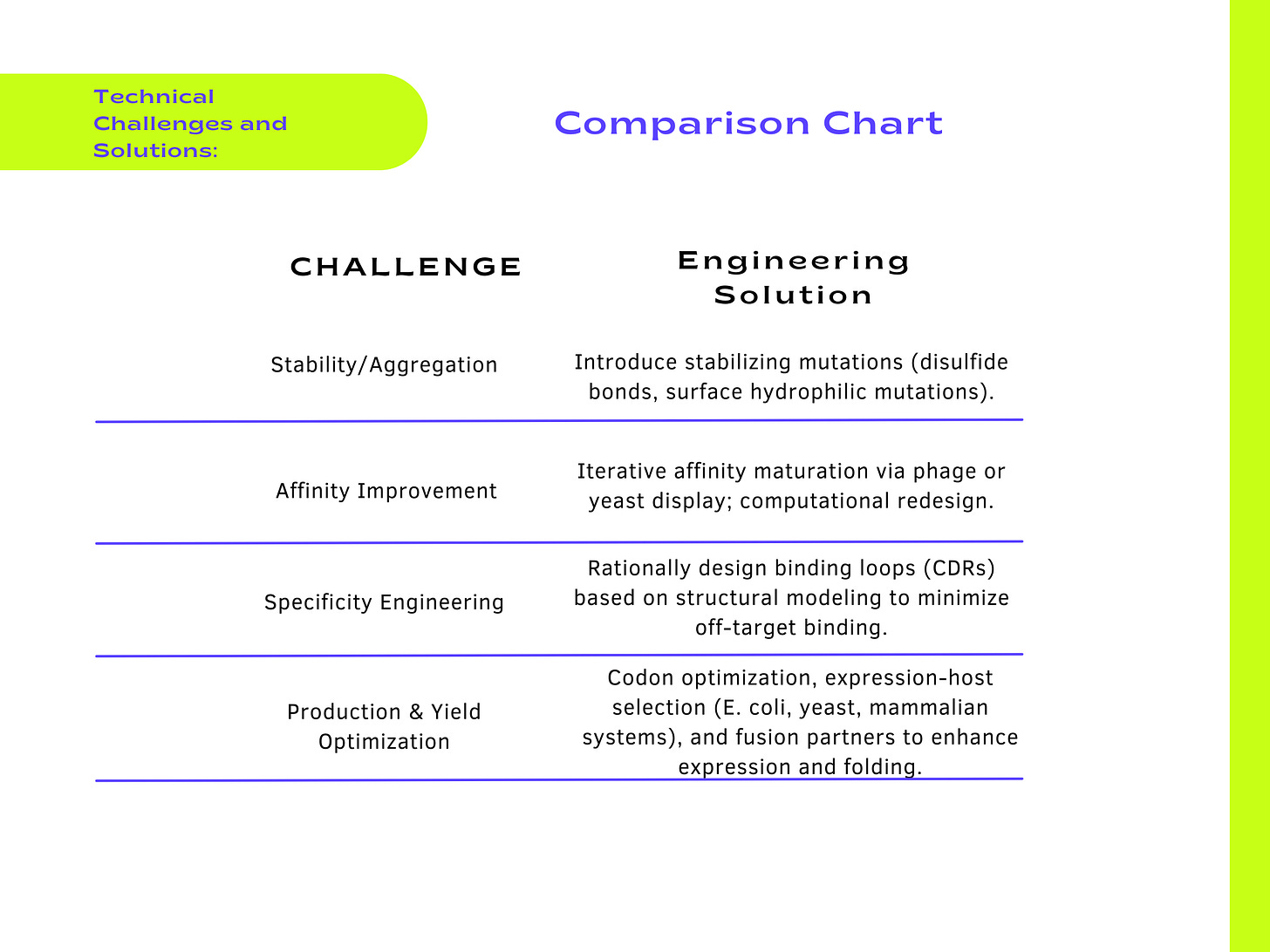
Despite their many advantages, antibody scaffolds face several challenges that must be addressed to fully realize their potential. One major issue is immunogenicity, particularly for scaffolds derived from non-human sources, such as camelid nanobodies. To mitigate this, researchers are humanizing these scaffolds or using computational tools to remove immunogenic epitopes. Another challenge is aggregation and poor solubility, which can limit therapeutic efficacy. Engineering hydrophilic surface residues or introducing stabilizing mutations, such as disulfide bonds, can improve solubility and reduce aggregation.
Future Directions and Innovations
Looking ahead, the field of antibody scaffold engineering is poised for even greater breakthroughs. Synthetic biology and de novo scaffold design are enabling the creation of entirely synthetic scaffolds with no natural counterparts, tailored for specific applications. These scaffolds can be designed with ultra-high stability and specificity, expanding their use beyond traditional therapeutic and diagnostic applications to include industrial enzymes and biosensors.
Antibody scaffolds are at the forefront of biomedical innovation, offering unparalleled flexibility, stability, and specificity for a wide range of applications. From multispecific and intracellular targeting to AI-driven design and advanced manufacturing, emerging trends are pushing the boundaries of what these scaffolds can achieve. While challenges remain, ongoing advancements in engineering and production are addressing these limitations, paving the way for the next generation of antibody-based therapies and diagnostics. As the field continues to evolve, antibody scaffolds will play an increasingly central role in precision medicine, transforming the way we diagnose, treat, and prevent diseases.
Conclusion
Antibody scaffolds have emerged as a transformative technology in biomedicine, offering a unique combination of specificity, stability, and versatility that addresses many of the limitations of traditional antibodies. By leveraging the fundamental antigen-binding regions of immunoglobulins in a minimized and engineered format, these scaffolds provide a powerful platform for targeting a wide range of biological molecules with precision. Their compact size enables enhanced tissue penetration and rapid clearance, while their modular design allows for extensive customization through advanced genetic engineering techniques such as site-directed mutagenesis, directed evolution, and computational modeling. These features have made antibody scaffolds indispensable tools in therapeutic development, diagnostics, and targeted drug delivery, with applications spanning oncology, autoimmune diseases, infectious diseases, and beyond.
The ongoing advancements in antibody scaffold engineering, including the development of multispecific and multivalent scaffolds, intracellular targeting strategies, and AI-driven design, are pushing the boundaries of what these molecules can achieve. Multispecific scaffolds, for instance, enable simultaneous targeting of multiple disease mediators, enhancing therapeutic efficacy in complex conditions like cancer and viral infections. Meanwhile, innovations in intracellular targeting are unlocking the potential to address previously "undruggable" intracellular proteins, such as oncogenic RAS and MYC, and to deliver gene-editing tools like CRISPR-Cas9 with unprecedented precision. The integration of artificial intelligence and machine learning into scaffold design is further accelerating the discovery and optimization of novel scaffolds, reducing development timelines and improving their functional properties.
Despite these remarkable advancements, challenges such as immunogenicity, aggregation, and rapid renal clearance remain areas of active research. Solutions such as humanization, stability-enhancing mutations, and conjugation to half-life extenders are being developed to overcome these hurdles, ensuring that antibody scaffolds can reach their full potential. Additionally, innovations in manufacturing, such as cell-free protein synthesis and continuous bioprocessing, are addressing the growing demand for scalable and cost-effective production methods, making these scaffolds more accessible for global health applications.
Looking ahead, the future of antibody scaffold technology is bright, with emerging trends such as synthetic biology, nanotechnology integration, and personalized medicine poised to drive further breakthroughs. The creation of entirely synthetic scaffolds, the conjugation of scaffolds with nanoparticles for enhanced therapeutic and diagnostic applications, and the development of patient-specific scaffolds tailored to individual disease profiles are just a few examples of the exciting directions this field is heading. As these innovations continue to unfold, antibody scaffolds will play an increasingly central role in precision medicine, transforming the way we diagnose, treat, and prevent diseases. Their molecular simplicity, engineering flexibility, and broad applicability ensure that they will remain at the forefront of biomedical innovation for years to come.

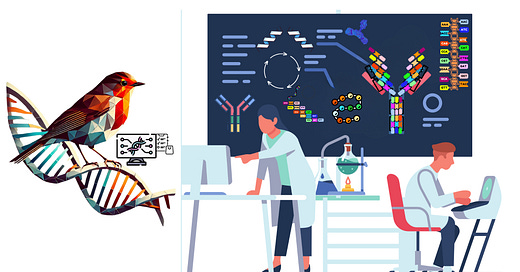

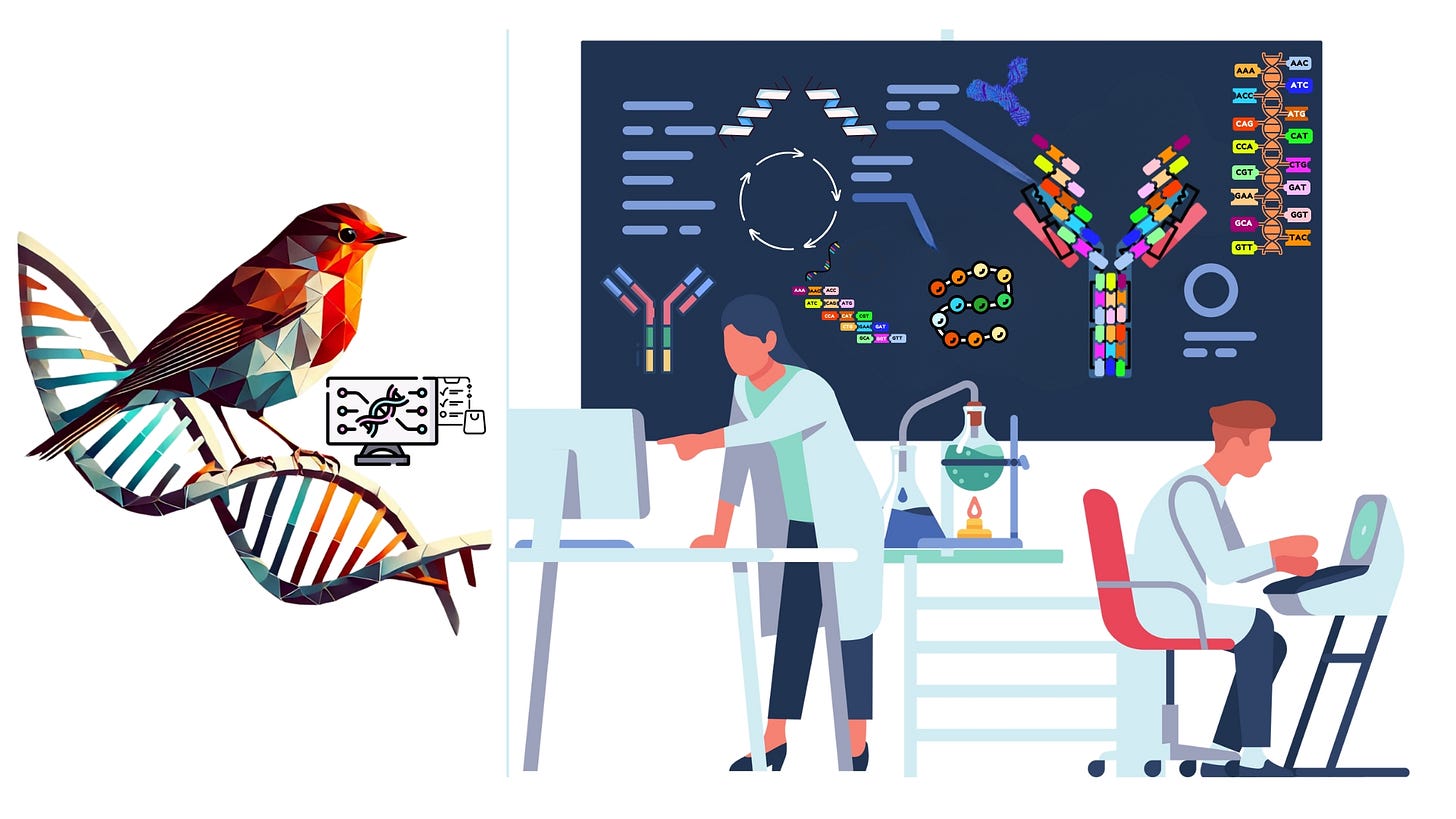
🧬 Support Independent Biotech Journalism 🧬
At BiotechnologyReviews.com, we’re committed to delivering in-depth, science-driven content that explores the cutting edge of genetics, molecular biology, and therapeutic innovation — all free and accessible to readers worldwide.
If you value high-quality, expertly researched articles on breakthroughs like epigenetic editing, gene therapy, and CRISPR-based technologies, we invite you to support our work.
Your pledge helps us:
Publish rigorous articles free from clickbait and hype
Cover underreported topics shaping the future of medicine and biotech
Keep our content independent, ad-light, and accessible to all
🔗 Make a difference — pledge your support today at https://lnkd.in/dDYUMY5g
Together, we can empower science-literate conversation and drive forward a more informed biotech future.