Epigenetic Editing: Precise Control of Gene Expression Without Altering DNA Sequence
Understanding How Gene Expression Can Be Precisely Controlled Without DNA Editing
Quick note: (if you are unfamiliar with any of the terms in this article, I have provided a helpful term glossary for you at the end)
Introduction
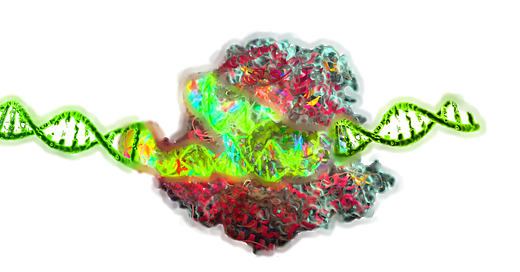
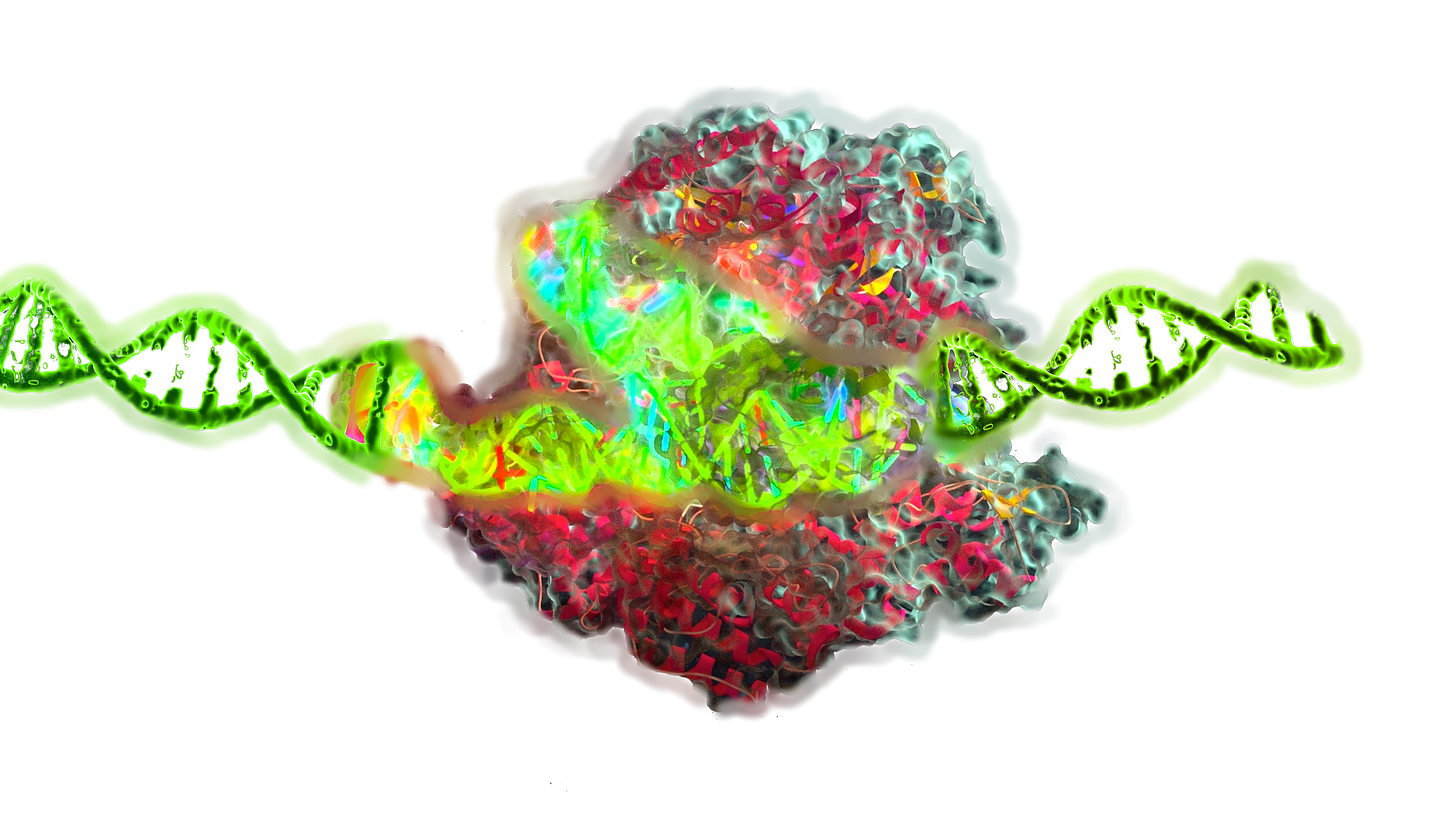
Epigenetic editing is an emerging class of genome engineering techniques that modulate gene expression without altering the underlying DNA sequence. Unlike traditional CRISPR Cas9 systems that induce double strand breaks (DSBs) and rely on error prone repair pathways, epigenetic editing uses catalytically inactive DNA binding platforms—such as dCas9, TALEs, or zinc fingers—fused to chromatin modifying effectors like DNMT3A, TET1, p300, KRAB, or LSD1. These chimeric tools enable precise and reversible modification of DNA methylation and histone post translational modifications (PTMs), altering transcriptional output at specific loci in a non mutagenic and often heritable manner.
This approach provides highly contextual control of gene regulation by targeting promoters, enhancers, and non coding regulatory elements in their native chromatin environment. Epigenetic editors allow for both gene activation and repression, supporting a range of applications from functional genomics and cellular reprogramming to therapeutic intervention. Key use cases include reactivation of epigenetically silenced genes in imprinting disorders (e.g., FMR1, UBE3A), silencing of oncogenes or viral elements (e.g., HPV, HIV), modulation of immune checkpoints (e.g., PD L1), and durable repression of disease causing alleles. The reversible nature of chromatin modifications enables temporal tuning of gene activity, while their mitotic stability supports long term effects without permanent genomic edits.
Advanced delivery systems—both viral (e.g., AAV, lentivirus) and non viral (e.g., LNPs, electroporation, RNPs)—are expanding the applicability of epigenetic editors across a variety of cell types and tissues. Innovations such as split intein reconstitution, barcoded sgRNAs, and optogenetic/chemical inducible systems offer control over spatial, temporal, and combinatorial aspects of gene regulation. Multiplexing strategies now allow parallel modulation of gene networks, enhancing the ability to study and reprogram complex cellular states. The development of epigenetic memory systems (e.g., CRISPRoff) and histone based switch circuits is also enabling synthetic biology applications, including lineage tracing and programmable cell behavior.
Compared to traditional genome editing, epigenetic editing offers superior safety, specificity, and regulatory precision. It avoids genotoxic effects, leverages endogenous regulatory architecture, allows allele specific targeting, and offers compatibility with post mitotic tissues and transient therapies. While challenges remain—including delivery efficiency, context dependent efficacy, and potential off target epigenomic remodeling—this field is rapidly progressing toward clinical applications. Epigenetic editing represents a paradigm shift from permanent sequence alteration to dynamic, context aware modulation of gene expression at single locus and systems levels.
Epigenetic Regulation Background
To understand epigenetic editing, it's necessary to understand the core mechanisms of epigenetic gene regulation:
DNA Methylation
The addition of methyl groups (CH₃) to the 5' carbon of cytosine residues, typically in CpG dinucleotides, is catalyzed by DNA methyltransferases (DNMTs). Methylation of promoter regions generally represses gene transcription.Histone Modifications
Histone proteins undergo a variety of post-translational modifications on their N-terminal tails, including:Acetylation (by HATs, histone acetyltransferases): usually associated with transcriptional activation.
Deacetylation (by HDACs, histone deacetylases): usually repressive.
Methylation (by histone methyltransferases): can activate or repress depending on the residue and methylation state (e.g. H3K4me3 is active, H3K9me3 is repressive).
Demethylation (by demethylases like LSD1 or JmjC domain proteins).
Chromatin Remodeling
Epigenetic changes alter nucleosome positioning and chromatin accessibility, influencing transcription factor binding and RNA polymerase recruitment.
DNA Methylation
DNA methylation refers to the covalent addition of a methyl group at the 5-carbon of cytosine rings, producing 5-methylcytosine (5mC). This occurs predominantly in the context of CpG dinucleotides in vertebrates. The reaction is catalyzed by DNA methyltransferases (DNMTs) using S-adenosylmethionine (SAM) as the methyl donor. Methylation is most dense in intergenic regions, repetitive elements, and gene bodies, whereas CpG islands—CG-rich promoter-associated regions—typically remain unmethylated in actively expressed genes. In addition to canonical CpG methylation, non-CpG methylation (e.g., CpA, CpT, CpC) is found in embryonic stem cells and neurons, where it may have regulatory functions.
DNMT1 acts as the maintenance methyltransferase, recognizing hemi-methylated DNA during replication and restoring symmetrical methylation. It is recruited to replication foci via UHRF1, which binds hemi-methylated CpGs and histone marks like H3K9me2.
DNMT3A and DNMT3B function as de novo methyltransferases, establishing new methylation patterns during early development and cellular differentiation.
DNMT3L is a catalytically inactive cofactor that stimulates DNMT3A/B activity by enhancing nucleosome binding and catalysis.
Functional roles of DNA methylation include long-term transcriptional silencing, genomic imprinting, transposon repression, and X-chromosome inactivation. Methylated DNA can directly inhibit transcription factor binding or recruit MBD (methyl-CpG-binding domain) proteins like MeCP2, which in turn assemble repressive complexes containing HDACs and histone methyltransferases.
Key points:
5mC is primarily deposited at CpG dinucleotides by DNMTs using SAM.
DNMT1 maintains methylation; DNMT3A/3B mediate de novo methylation.
Methylation represses transcription by occluding TFs and recruiting repressors.
Histone Modifications
Histone post-translational modifications (PTMs) are chemical alterations to histone tails that alter chromatin structure and regulate gene expression. These modifications include acetylation, methylation, phosphorylation, ubiquitination, and more. Modifications occur primarily on the N-terminal tails of histone H3 and H4, but also on H2A and H2B. Acetylation of lysine residues, mediated by histone acetyltransferases (HATs) such as p300/CBP and GCN5, neutralizes the positive charge on lysines, thereby decreasing histone-DNA affinity and enhancing chromatin accessibility. This is typically associated with active transcription, especially marks like H3K9ac and H3K27ac.
Histone methylation is more nuanced. It is catalyzed by SET domain-containing methyltransferases, often specific to particular lysine residues. For example, H3K4me3 is found at active promoters, H3K36me3 marks gene bodies during elongation, and H3K27me3 and H3K9me3 are hallmarks of repressive chromatin. Methylation states (mono-, di-, tri-methylation) further specify chromatin state. Demethylases fall into two families: LSD1/KDM1A, which uses an FAD-dependent oxidative mechanism, and JmjC-domain proteins, which are Fe(II)/α-ketoglutarate-dependent dioxygenases capable of removing all methylation states.
PTMs are interpreted by "reader" proteins:
Bromodomains bind acetylated lysines.
Chromodomains bind methylated lysines.
PHD fingers often interpret combinatorial modifications.
These interactions facilitate the assembly of transcriptional activator or repressor complexes at specific genomic loci.
Key points:
Acetylation promotes gene activation; deacetylation represses it.
Histone methylation can activate (e.g., H3K4me3) or repress (e.g., H3K27me3).
PTMs are interpreted by chromatin reader domains to mediate gene regulation.
Chromatin Remodeling and Structure
Beyond chemical modifications, chromatin structure is regulated by ATP-dependent chromatin remodeling complexes that reposition, evict, or restructure nucleosomes. These remodelers include SWI/SNF, ISWI, CHD, and INO80 families. They typically contain a catalytic ATPase subunit that uses ATP hydrolysis to disrupt histone-DNA contacts, thereby modulating the accessibility of DNA to transcription factors, polymerases, and repair machinery.
Remodelers influence nucleosome occupancy, spacing, and histone variant incorporation (e.g., H2A.Z, H3.3), all of which affect chromatin dynamics and transcription. Remodeling is critical for transitions between euchromatin (open, transcriptionally active) and heterochromatin (condensed, repressive). Constitutive heterochromatin is characterized by repetitive sequences and H3K9me3 enrichment, recruiting HP1 proteins to mediate chromatin compaction. In contrast, facultative heterochromatin involves gene silencing during differentiation or development and is marked by H3K27me3 catalyzed by EZH2 of the Polycomb Repressive Complex 2 (PRC2).
Key points:
Remodelers alter nucleosome position and composition using ATP hydrolysis.
Constitutive heterochromatin (H3K9me3/HP1) is stably repressive.
Facultative heterochromatin (H3K27me3/PRC2) is developmentally regulated.
Integration of Epigenetic Layers
Epigenetic regulation is not modular but highly interconnected, involving extensive cross-talk between DNA methylation, histone PTMs, and chromatin remodeling. For instance, DNMTs interact with H3K9 methyltransferases (e.g., G9a/SUV39H1) to reinforce silencing via mutual recruitment. UHRF1, a key regulator of DNMT1, contains both a SET- and RING-associated (SRA) domain that binds hemi-methylated CpGs and a tandem Tudor domain that binds methylated histones. This ensures coordination between DNA methylation and histone methylation.
Polycomb group complexes, particularly PRC2, preferentially bind to CpG-rich, unmethylated DNA and deposit H3K27me3. These regions can be further compacted by PRC1, which ubiquitinates H2AK119 and mediates chromatin looping. In contrast, active enhancers are marked by H3K27ac and show high chromatin accessibility. Three-dimensional chromatin structure, mediated by CTCF, cohesin, and other architectural proteins, further modulates enhancer-promoter interactions and establishes topologically associating domains (TADs).
Key points:
Epigenetic regulators are biochemically and functionally interconnected.
UHRF1 links histone methylation and DNA methylation during replication.
PRC2 targets unmethylated CpG islands to establish facultative heterochromatin.
Epigenetic Editing: Core Principles
Epigenetic editing relies on programmable DNA-binding platforms that localize epigenetic effector domains to specific genomic loci. The goal is to modify the epigenetic state in a locus-specific, reversible, and non-mutagenic manner.
DNA-Binding Platforms
Several types of sequence-specific DNA-binding domains are used:
dCas9: A catalytically dead form of Cas9 (from CRISPR systems) that retains DNA-binding ability via a guide RNA (gRNA), but lacks endonuclease activity.
Zinc Finger Proteins (ZFPs): Engineered proteins that recognize specific DNA triplets.
Transcription Activator-Like Effectors (TALEs): Modular proteins recognizing single DNA bases via repeat-variable diresidues (RVDs).
Effector Domains
These are epigenetic enzymes or proteins that are fused to the DNA-binding domains:
DNMT3A or DNMT3A–DNMT3L fusion: for targeted DNA methylation.
TET1 catalytic domain: for targeted DNA demethylation via oxidation of 5-methylcytosine.
p300 core domain: histone acetyltransferase for H3K27ac enrichment and transcriptional activation.
KRAB (Krüppel-associated box): a repressor domain that recruits heterochromatin machinery such as KAP1 and HP1.
LSD1 or KDM1A: histone demethylase used to remove activating methyl marks like H3K4me2.
EZH2: the catalytic subunit of PRC2 complex, adds H3K27me3 (repressive mark).
Programmable DNA-Binding Platforms
The foundation of epigenetic editing lies in programmable, sequence-specific DNA-binding platforms that allow precise localization of effector domains to defined genomic loci. The three principal platforms currently in use are:
Catalytically inactive Cas9 (dCas9): A nuclease-dead mutant of Cas9 in which the RuvC and HNH nuclease domains are inactivated (usually via D10A and H840A mutations). dCas9 retains its ability to bind DNA in a sequence-specific manner via a single guide RNA (sgRNA), without inducing double-strand breaks. The sgRNA contains a 20-nucleotide spacer that directs the dCas9 complex to the complementary target DNA sequence adjacent to a protospacer adjacent motif (PAM), typically NGG for S. pyogenes Cas9.
Zinc Finger Proteins (ZFPs): Engineered DNA-binding proteins composed of tandemly arrayed C2H2 zinc finger domains. Each zinc finger domain recognizes a specific 3-bp DNA sequence via contacts in the major groove, allowing modular assembly of ZFPs to target longer genomic regions (typically 9–18 bp).
Transcription Activator-Like Effectors (TALEs): Derived from Xanthomonas bacteria, TALEs consist of tandem repeats (~34 amino acids each), each recognizing a single base pair through two hypervariable residues at positions 12 and 13, known as repeat-variable diresidues (RVDs). The DNA-targeting specificity is determined by the RVD composition and repeat order.
Each platform has distinct trade-offs in terms of modularity, off-target risk, delivery vector size, and ease of design. dCas9-based systems are the most widely used due to their scalability and simplicity in retargeting via RNA rather than protein engineering.
Key points:
dCas9 uses RNA-guided targeting without DNA cleavage.
ZFPs and TALEs are modular DNA-binding proteins with base-specific recognition.
Each platform enables locus-specific recruitment of effector domains.
Epigenetic Effector Domains
Epigenetic effectors are functional protein domains that enzymatically modify chromatin without altering the DNA sequence. When fused to DNA-targeting platforms, they enable precise modification of epigenetic marks. The major classes of effectors include:
DNA methylation effectors:
DNMT3A catalytic domain: Catalyzes de novo methylation of unmethylated CpG sites. Often fused with DNMT3L, a regulatory cofactor that increases substrate affinity and cooperativity.
TET1 catalytic domain: Mediates active DNA demethylation by iterative oxidation of 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC), then to 5-formylcytosine (5fC), and 5-carboxylcytosine (5caC), which are removed by thymine DNA glycosylase (TDG) and base excision repair.
Histone modification effectors:
p300 HAT core domain: Acetylates histone H3 at lysine 27 (H3K27ac), associated with enhancer and promoter activation. Local enrichment of H3K27ac recruits BRD-containing transcriptional coactivators.
LSD1/KDM1A: FAD-dependent amine oxidase that demethylates mono- and di-methylated H3K4 (H3K4me1/2), thereby reducing active chromatin signatures.
EZH2 (PRC2): Methylates H3K27 to form H3K27me3, a repressive mark that promotes facultative heterochromatin.
G9a: Catalyzes mono- and di-methylation of H3K9, another repressive chromatin mark.
Transcriptional regulatory effectors:
KRAB (Krüppel-associated box): A potent transcriptional repressor that recruits KAP1/TRIM28, which in turn recruits SETDB1 (H3K9 methyltransferase), HP1, and the NuRD complex (HDACs + chromatin remodelers) to induce heterochromatin formation.
VP64, VPR: Synthetic transcriptional activators that function via recruitment of general transcription factors, Mediator complex, and chromatin-modifying coactivators.
Key points:
Effector domains include DNA methylators (DNMT3A), demethylators (TET1), HATs (p300), HDACs, methyltransferases (EZH2, G9a), and repressors (KRAB).
Specificity depends on both the binding platform and the epigenetic domain fused.
Effects can be transcriptionally activating or repressive, depending on effector class.
Fusion Protein Design and Expression
Functional epigenetic editors are constructed as chimeric proteins or modular complexes composed of a DNA-binding domain (e.g., dCas9) and an epigenetic effector domain linked via a flexible peptide linker, typically a glycine-serine (Gly-Ser) repeat motif to reduce steric hindrance and maintain independent domain function. The overall architecture must preserve nuclear localization signals (NLS), DNA binding fidelity, catalytic activity of the effector, and chromatin access.
The method of delivery and expression system impacts efficiency:
Transient expression: mRNA, plasmids, or protein-RNP complexes for short-term editing.
Stable expression: Lentiviral or transposon-based delivery for long-term chromatin remodeling.
Inducible expression: Doxycycline- or tamoxifen-inducible promoters, optogenetic control, or split-protein systems enable temporal control over editing activity.
Design considerations also include codon optimization, degradation tags (e.g., PEST domains for turnover), and multi-effector scaffolds (e.g., SunTag or MS2 systems) to amplify the effect via multimerized effectors.
Key points:
Fusion proteins require careful linker design and nuclear localization.
Expression methods include transient, stable, or inducible systems.
Multi-component systems can increase signal strength and specificity.
Specificity and Targeting Parameters
Achieving locus-specific epigenetic editing requires controlling both the sequence specificity of the DNA-binding domain and the context-specific activity of the effector. For CRISPR/dCas9-based systems:
Target specificity depends on perfect complementarity of the 20-nt sgRNA to the DNA target and the presence of a compatible PAM sequence.
Off-target activity can arise from partial complementarity, particularly in the seed region (proximal to PAM), or excessive effector activity diffusing beyond the target locus.
To improve specificity:
Truncated sgRNAs (17–18 nt) reduce off-target binding.
High-fidelity Cas9 variants (e.g., eSpCas9, HF1, HypaCas9) reduce non-specific DNA binding.
Split effector systems (e.g., dCas9-FKBP + FRB-effector, inducible by rapamycin) restrict activity to dimerization events at target loci.
Chromatin accessibility also influences targeting. Closed heterochromatin can impair dCas9 binding, which can be partially mitigated by co-recruitment of pioneer factors or chromatin remodelers.
Key points:
Specificity is determined by sgRNA sequence, PAM compatibility, and chromatin state.
Strategies to enhance specificity include truncated guides, high-fidelity variants, and split effectors.
Epigenetic context (e.g., heterochromatin) can limit target accessibility.
Targeting Strategy and Delivery
To target an epigenetic modification, a fusion protein is designed that combines a DNA-targeting domain (e.g. dCas9 or TALE) with an epigenetic effector. Delivery can be accomplished via:
Plasmid transfection
Viral vectors (e.g. lentivirus, AAV)
Ribonucleoprotein (RNP) complexes
mRNA delivery
The choice depends on cell type, efficiency requirements, and duration of expression.
Locus-Specific Targeting Considerations
The fundamental requirement of epigenetic editing is precise localization of the editing complex to the intended genomic locus. In dCas9-based systems, targeting is achieved via a single guide RNA (sgRNA) that hybridizes to a 20-nucleotide DNA sequence, directly upstream of a Protospacer Adjacent Motif (PAM)—usually 5′-NGG for S. pyogenes Cas9. Target specificity is determined largely by the 5′ “seed region” (first 8–12 bp adjacent to PAM), where mismatches significantly reduce binding affinity. However, Cas9 tolerates mismatches in distal regions, allowing potential off-target occupancy. The chromatin context of the target—particularly nucleosome occupancy, DNA methylation, and histone modifications—directly affects the accessibility and binding efficiency of the editing complex.
In the case of ZFP and TALE-based systems, specificity is encoded at the protein level. ZFPs recognize triplets of bases, while TALEs recognize single bases. However, both require careful assembly of modular domains and may suffer from off-target interactions due to partial motif degeneracy or binding to noncanonical DNA structures (e.g., G-quadruplexes). For all platforms, chromatin accessibility is a limiting factor, particularly in repressed genomic regions marked by H3K9me3 or dense DNA methylation. Access to these regions can be enhanced by co-delivery of chromatin remodelers (e.g., BRG1, SNF2H) or pioneer factors (e.g., FOXA1).
Key points:
dCas9 target recognition is PAM- and seed-region-dependent.
Off-target risk arises from distal mismatches and chromatin accessibility.
TALE/ZFP systems require precise protein engineering and are influenced by DNA structure.
Closed chromatin impairs binding; remodelers can enhance accessibility.
Delivery Modalities: DNA, RNA, and Protein
Delivery of epigenetic editing components can be achieved at the level of DNA, mRNA, or protein, with each route having distinct pharmacokinetic and biological properties.
Plasmid DNA delivery (e.g., via lipofection or electroporation) enables high levels of expression but risks genomic integration and prolonged exposure, which may increase off-target effects and cellular stress responses. Promoters such as CMV, EF1α, or CAG are commonly used for constitutive expression, but inducible promoters (e.g., Tet-On, Cre/loxP) allow for controlled activation.
mRNA delivery (e.g., via lipid nanoparticles or electroporation) provides transient expression without risk of genomic integration. In vitro transcribed mRNA must be chemically stabilized (e.g., pseudouridine and 5-methylcytidine substitution, anti-reverse cap analogs) and often includes a 5′ cap, 3′ poly(A) tail, and untranslated regions (UTRs) for translation efficiency and stability.
Protein delivery, typically as ribonucleoprotein (RNP) complexes (e.g., dCas9 + sgRNA), offers the most rapid and transient editing with minimal cellular burden. However, proteins must be stabilized (e.g., via fusion to cell-penetrating peptides like TAT, or encapsulation in polymers or nanoparticles) and protected from proteolytic degradation. Nuclear localization signals (NLS) must be intact for effective chromatin targeting.
Key points:
DNA delivery is efficient but may be prolonged and integrate.
mRNA delivery is transient and safer, requiring stabilization chemistry.
RNP delivery is fast, transient, and integration-free but limited by protein stability and delivery efficiency.
Viral Delivery Vectors
Viral vectors offer high-efficiency delivery and can accommodate long-term expression in dividing and non-dividing cells. The choice of vector is governed by payload size, tissue tropism, immunogenicity, and regulatory concerns.
Lentiviral vectors (LVs): Based on HIV-1, they allow for stable genomic integration in both dividing and non-dividing cells. They can accommodate ~8–9 kb payloads and are pseudotyped with VSV-G for broad tropism. LVs are useful for generating stable cell lines expressing dCas9-effector fusions, but integration raises risks of insertional mutagenesis and long-term genomic perturbation.
Adeno-associated virus (AAV): AAV vectors are non-integrating and episomal, offering safer long-term expression in vivo. However, their packaging capacity is limited (~4.7 kb), which typically precludes the inclusion of both dCas9 and large effector domains. This limitation is overcome using dual-vector systems or split-intein strategies to reconstitute large proteins intracellularly.
Adenoviral vectors: High-capacity (up to ~36 kb), non-integrating, and highly immunogenic, making them suitable for short-term in vivo applications where transient, high-level expression is needed.
Integration-deficient lentiviruses (IDLVs) and self-inactivating (SIN) LVs reduce genomic integration risks by mutating integrase or deleting LTRs, respectively.
Key points:
Lentiviruses support stable integration and large payloads; risk insertional mutagenesis.
AAVs are safer but limited in capacity; workarounds include dual vectors.
Adenoviruses allow high expression but are more immunogenic.
IDLVs and SIN-LVs mitigate integration risks.
Non-Viral Delivery Systems
Non-viral strategies offer lower immunogenicity and better control over transient expression but often suffer from reduced efficiency, particularly in vivo.
Lipid nanoparticles (LNPs): Cationic or ionizable lipids form complexes with mRNA, DNA, or RNPs, facilitating endocytosis and endosomal escape. LNPs are the most clinically advanced non-viral delivery system and have been used for mRNA vaccines and Cas9 delivery. Key components include ionizable lipids (e.g., DLin-MC3-DMA), cholesterol, PEG-lipids, and helper lipids.
Electroporation: High-voltage pulses transiently permeabilize the membrane to allow nucleic acid/protein entry. High efficiency in ex vivo applications (e.g., CAR-T cell engineering), but limited to suspension cells and can cause significant cytotoxicity.
Polymeric nanoparticles: Polymers like PEI, PLGA, or dendrimers complex with nucleic acids or proteins. Surface functionalization (e.g., targeting ligands, PEGylation) enhances biodistribution and endosomal escape.
Gold nanoparticles, silica nanoparticles, and cell-penetrating peptides are also under development for epigenetic tool delivery but are less mature in clinical applications.
Key points:
LNPs are clinically validated for RNA and RNP delivery.
Electroporation is efficient ex vivo but cytotoxic.
Polymers and nanomaterials offer modularity but require tuning for stability and targeting.
Multicomponent and Modular Systems
Advanced targeting strategies often involve multi-component scaffolding systems to enhance potency, modularity, or control.
SunTag system: A tandem repeat array of GCN4 peptides fused to dCas9 enables recruitment of multiple scFv-fused effectors (e.g., p300, VP64). This amplifies local chromatin modification and increases editing efficiency.
MS2/PP7 systems: Stem-loop sequences are appended to the sgRNA, which are bound by coat proteins (MCP/PCP) fused to effectors. This enables RNA-guided multimerization of epigenetic effectors without modifying the DNA-binding protein.
Split dCas9 or split effector systems: Inducible by light (e.g., CRY2-CIB1) or small molecules (e.g., rapamycin-FKBP/FRB), allowing spatiotemporal control over editing activity.
Barcoding and multiplexing: Use of orthogonal Cas9 variants (e.g., S. aureus, N. meningitidis) or barcoded sgRNA scaffolds enables simultaneous targeting of multiple loci with different effectors.
Key points:
SunTag and MS2 systems allow amplification and modularity.
Split systems offer inducible control of activity.
Multiplexing enables combinatorial epigenetic programming at scale.
Applications
Gene Repression
Using dCas9-KRAB or dCas9-DNMT3A to silence endogenous genes. This is useful for:
Functional genomics (loss-of-function studies)
Repressing disease-related genes
Inducing long-term silencing via heterochromatin formation
Gene Activation
Using dCas9-p300 or dCas9-VP64 to activate transcription of endogenous genes:
Cellular reprogramming
Inducing tumor suppressor gene expression
Reactivating fetal hemoglobin in hemoglobinopathies
Epigenetic Memory
Unlike transient transcription factor binding, certain epigenetic changes (e.g., DNA methylation) can persist through cell division, offering stable but potentially reversible gene regulation.
Targeted Gene Silencing
One of the primary applications of epigenetic editing is stable transcriptional repression of specific endogenous genes without inducing DNA double-strand breaks (DSBs). Repressive effectors such as KRAB, DNMT3A, G9a, and EZH2 can be targeted to promoter or enhancer regions via dCas9, TALEs, or ZFPs.
KRAB recruits the KAP1/TRIM28 scaffold protein, which then assembles a multi-protein repressive complex that includes SETDB1 (H3K9me3 methyltransferase), HP1 (heterochromatin protein 1), and the NuRD complex (containing HDAC1/2 and CHD3/4). This results in local heterochromatin formation and durable transcriptional silencing.
DNMT3A or DNMT3A-DNMT3L fusions catalyze CpG methylation at gene promoters, often inducing long-term repression that is maintained across cell divisions via DNMT1 recruitment during DNA replication.
G9a catalyzes H3K9me2 deposition, establishing a repressive chromatin domain that can also recruit HP1α.
Gene repression is especially relevant for:
Functional genomic screens (loss-of-function studies)
Inhibition of disease-causing alleles (e.g., gain-of-function mutations)
Silencing of viral genes (e.g., latent HIV-1 provirus)
Key points:
KRAB recruits heterochromatin-forming complexes via KAP1.
DNMT3A enables mitotically stable gene silencing via promoter methylation.
H3K9me2 and H3K27me3 deposition enforce facultative repression.
Gene Activation and Enhancer Reprogramming
Epigenetic editing is also used to induce targeted transcriptional activation of silent or lowly expressed genes through the recruitment of activating chromatin marks or transcriptional coactivators.
p300 core domain (a histone acetyltransferase) catalyzes the acetylation of H3K27, a hallmark of active enhancers and promoters. Local H3K27ac enrichment facilitates recruitment of bromodomain-containing proteins (e.g., BRD4), Mediator complex, and RNA Pol II, promoting transcription initiation.
TET1 catalytic domain can reverse CpG methylation at silenced promoters, thereby enabling reactivation of epigenetically silenced genes.
Synthetic transcriptional activators (e.g., dCas9-VP64, dCas9-VPR) directly recruit basal transcriptional machinery and chromatin remodelers.
Multiplexed activation of multiple loci (e.g., lineage-determining TFs) enables cell fate reprogramming or transdifferentiation without integration of exogenous cDNA.
Applications include:
Reprogramming somatic cells into induced pluripotent stem cells (iPSCs) or other lineages.
Reactivation of fetal hemoglobin (HbF) in β-hemoglobinopathies via BCL11A enhancer modulation.
Endogenous gene rescue in haploinsufficiency or loss-of-function disorders.
Key points:
p300 promotes H3K27ac and enhancer activation.
TET1 removes promoter methylation to restore gene activity.
Activator domains like VP64 recruit the transcriptional machinery directly.
Therapeutic Reactivation of Endogenous Genes
Certain diseases arise from the epigenetic silencing of genes without underlying DNA mutations. Epigenetic editing enables reactivation of endogenously silenced genes in a sequence-specific and heritable (yet reversible) manner, avoiding global demethylation or HDAC inhibition.
Examples include:
Fragile X Syndrome: Reactivation of FMR1, which is silenced via CGG repeat expansion and promoter hypermethylation. TET1 or dCas9-VPR systems targeted to the FMR1 promoter have been shown to partially restore expression in patient-derived neurons.
Angelman Syndrome: Loss of maternal UBE3A due to imprinting. Silencing of the UBE3A-ATS antisense transcript via dCas9-KRAB enables unsilencing of the paternal allele.
Laminopathies (e.g., Hutchinson-Gilford Progeria Syndrome): Mutant LMNA expression can be repressed via dCas9-KRAB, targeting cryptic splice sites or regulatory elements.
This category of applications represents a paradigm shift from mutation correction to allelic rebalancing via epigenetic control.
Key points:
Epigenetic editing reactivates silenced loci without altering DNA sequence.
Applications include imprinting disorders and repeat-associated gene silencing.
Allele-specific control is feasible by targeting polymorphisms or methylation patterns.
Disease Modeling and Functional Genomics
Epigenetic editing tools are essential in functional genomics for elucidating the role of non-coding regulatory elements, such as enhancers, silencers, and insulators, that cannot be disrupted with conventional coding mutations.
dCas9-p300 or dCas9-KRAB can be used to modulate enhancer activity and assess its impact on target gene expression via chromatin conformation capture (e.g., 4C, HiChIP).
Editing of super-enhancers, defined by broad H3K27ac domains and high Mediator occupancy, allows interrogation of transcriptional control in oncogenes (e.g., MYC, BCL2).
Perturbation of insulator elements (e.g., CTCF-bound boundaries) allows testing of TAD insulation in 3D genome structure.
Epigenetic editing of long non-coding RNAs (lncRNAs) and antisense RNAs can distinguish cis- vs. trans-regulatory functions.
High-throughput versions of these approaches (e.g., CRISPRi/a screens) can identify regulatory elements involved in lineage specification, drug resistance, or disease phenotypes.
Key points:
Enables perturbation of non-coding regulatory elements with base-pair precision.
Super-enhancer and insulator editing helps decode 3D genome regulation.
Functional dissection of lncRNA loci is achievable via transcriptional repression or activation.
Epigenetic Memory and Cellular Reprogramming
A unique advantage of epigenetic editing is the induction of stable yet reversible cellular states. Chromatin modifications—particularly DNA methylation and H3K9me3/H3K27me3 deposition—can persist across cell divisions, creating long-lived changes in gene expression even after the removal of the editing machinery.
Epigenetic memory is useful in stem cell programming, where transient exposure to dCas9-based editors can induce sustained reprogramming without permanent genomic alterations.
Stable repression of differentiation inhibitors (e.g., REST, ID2) or activation of lineage-defining transcription factors (e.g., MYOD1, GATA4) can drive cells toward specific fates.
In cancer biology, durable silencing of oncogenes or reactivation of tumor suppressors may reduce tumorigenic potential or sensitize cells to therapy.
These effects are being explored in ex vivo engineered cell therapies, such as T cells or iPSCs with reprogrammed epigenomes, as well as in in vivo programming for regenerative medicine.
Key points:
Epigenetic marks can persist through mitosis, conferring “memory” of gene expression state.
Useful for long-term reprogramming of cell identity without genomic editing.
Has applications in stem cell engineering, immunotherapy, and cancer biology.
Immunomodulation and Viral Latency Reversal
Targeted epigenetic modulation has therapeutic potential in host-pathogen interactions, especially where pathogens hijack or become embedded within the host epigenome.
Latent HIV-1 proviruses are embedded in heterochromatin and silenced. Latency reversal can be achieved by targeting the viral LTR with dCas9-p300 or VP64, reactivating transcription for “shock-and-kill” therapeutic strategies.
In contrast, endogenous retroelements and oncogenic viral genes (e.g., HPV E6/E7) can be silenced via dCas9-KRAB or DNMT3A targeting to reduce pathogenic gene expression.
Immune checkpoint genes (e.g., PD-L1) can be repressed or enhanced to fine-tune immune responses in T cells or tumor cells.
These approaches enable precision immunomodulation without the global immunosuppressive effects of traditional drugs.
Key points:
Latent virus activation (HIV) or repression (HPV) is possible via epigenetic targeting.
Checkpoint modulation (e.g., PD-L1) can improve immunotherapy.
Epigenetic tools allow precise immune reprogramming with minimal off-target effects.
Advantages Over Traditional CRISPR
No double-strand breaks (DSBs) → reduces risk of off-target mutations and genotoxicity.
Reversibility → many epigenetic modifications are reversible with time or further intervention.
Modular design → combinations of DNA-binding and effector domains enable diverse regulatory outcomes.
Locus specificity → targets individual genes or regulatory elements without altering global chromatin.
Non-Disruptive, Reversible Mechanism of Action
Traditional CRISPR-Cas9 induces DNA double-strand breaks (DSBs), which are then repaired via non-homologous end joining (NHEJ) or homology-directed repair (HDR). Both pathways carry intrinsic risks: NHEJ is error-prone and can result in insertions, deletions (indels), or translocations; HDR is inefficient in most somatic cells and restricted to S/G2 phase. In contrast, epigenetic editing employs dCas9 (or similar DNA-binding platforms) that retain DNA-targeting capability but lack nuclease activity, thereby preserving genomic sequence integrity.
Furthermore, the epigenetic modifications induced—such as DNA methylation, histone acetylation, or methylation—are potentially reversible via cellular enzymes (e.g., TET demethylases, HDACs, demethylases) or by engineered effectors (e.g., dCas9-TET1, dCas9-LSD1). This enables modulation of gene expression without permanent alteration to the genetic code. Such reversibility is particularly valuable in therapeutic contexts where transient gene control is needed or safety concerns preclude permanent genome edits.
Key points:
Epigenetic editing does not induce DSBs, avoiding indels and chromosomal rearrangements.
Modifications are reversible via endogenous or synthetic enzymes.
Suitable for temporary or conditionally regulated gene expression changes.
Contextual and Regulatory Precision
Epigenetic editing enables precise modulation of gene expression in its endogenous genomic and chromatin context, preserving cis-regulatory architecture, epigenetic insulation, and native 3D chromatin topology. Unlike CRISPR-based coding sequence edits, which may bypass or distort natural regulation (e.g., overexpression from exogenous promoters), epigenetic editors allow for modulation of gene expression amplitude via alterations to promoters, enhancers, silencers, or insulator elements.
This native-context targeting is especially important for:
Developmental genes, which require tight spatiotemporal regulation.
Dosage-sensitive loci, where precise expression levels are critical (e.g., transcription factors, signaling proteins).
Non-coding elements, whose perturbation would be poorly modeled by sequence deletion alone.
Moreover, tissue-specific chromatin states influence effector action, enabling cell-type-selective activity. For example, dCas9-DNMT3A may induce silencing only at accessible promoters, limiting off-target activity in closed chromatin.
Key points:
Enables gene modulation within endogenous regulatory context.
Suitable for non-coding regions and dosage-sensitive loci.
Chromatin state-dependent activity provides intrinsic cell-type specificity.
Allele-Specific and Imprinting-Compatible Targeting
One of the unique advantages of epigenetic editing is its potential for allele-specific targeting, critical for diseases involving dominant-negative mutations, imprinting disorders, or X-linked diseases. Unlike traditional CRISPR, which typically edits both alleles indiscriminately, epigenetic editors can exploit cis-acting differences (e.g., SNPs, DNA methylation, allele-specific chromatin marks) to selectively modify the pathogenic allele.
This is particularly relevant in:
Fragile X Syndrome, where only the hypermethylated, silenced FMR1 allele requires activation.
Prader-Willi and Angelman syndromes, which involve loss-of-function or silencing of imprinted loci.
X-chromosome-linked diseases, where reactivation of the inactive X allele (e.g., MECP2) in females can be beneficial.
Such allele-specific epigenetic control is extremely difficult to achieve with conventional CRISPR due to the high sequence homology between alleles.
Key points:
Enables selective modulation of one allele while preserving the other.
Applicable to imprinting disorders and X-linked diseases.
Takes advantage of allele-specific methylation or SNPs for discrimination.
Reduced Immunogenicity and DNA Damage Response Activation
CRISPR-induced DSBs elicit a robust DNA damage response (DDR) involving activation of ATM/ATR kinases, γH2AX, and p53 pathways. In certain cell types (e.g., hematopoietic stem cells, neurons), this can trigger apoptosis, cell cycle arrest, or senescence, severely compromising editing efficiency and cell viability. Moreover, persistent expression of Cas9 protein has been shown to elicit adaptive immune responses, particularly in pre-exposed individuals due to widespread Streptococcus pyogenes colonization.
Epigenetic editing circumvents both issues:
No DSBs, therefore no DDR activation.
Transient delivery of dCas9-RNPs or mRNA limits immune detection.
Use of orthologous or humanized effectors (e.g., KRAB, p300) reduces risk of immunogenicity compared to bacterial proteins.
This makes epigenetic editing particularly attractive for in vivo therapeutic applications, where immune responses and DNA damage are major limiting factors.
Key points:
No activation of DNA damage sensors (ATM/ATR/p53).
Reduced cytotoxicity and improved viability in sensitive cell types.
Lower immunogenicity via transient expression and non-bacterial domains.
Epigenetic Memory and Long-Term Modulation Without Integration
One of the most clinically significant features of epigenetic editing is the persistence of chromatin modifications, even in the absence of continued effector expression. For instance, DNA methylation induced by dCas9-DNMT3A can be propagated through DNA replication via recruitment of DNMT1, maintaining silencing across multiple cell divisions. Similarly, KRAB-mediated H3K9me3 or EZH2-mediated H3K27me3 can lead to facultative heterochromatin formation, maintained through reader-writer feedback loops involving HP1 and PRC2 complexes, respectively.
Unlike CRISPR-HDR, which may require integration of donor templates or sustained selection pressure for maintenance, epigenetic editing achieves long-term gene modulation without foreign DNA insertion or continuous transgene expression. This is crucial for:
Ex vivo cell therapies (e.g., T cells, iPSCs) where persistent editing is desired but integration is unsafe.
Neuronal and muscle tissues, where cell turnover is low and durable effects are favored.
Key points:
DNA methylation and repressive histone marks can persist through cell division.
Long-term gene control achievable without integration.
Useful in post-mitotic cells or long-lived lineages.
Multiplexing and Combinatorial Control of Gene Networks
Epigenetic editing platforms can be multiplexed to target multiple genes or regulatory elements simultaneously, enabling network-level modulation. This is more scalable and tunable than traditional CRISPR knockout or knock-in strategies, which typically require independent design and delivery per locus.
Using systems like:
dCas9 + MS2/PP7 scaffold RNAs, each sgRNA can recruit a unique effector domain, allowing orthogonal activation/repression.
CRISPRa/i pooled screens, libraries of guide RNAs are used to screen regulatory elements genome-wide.
SunTag arrays allow signal amplification at single loci by recruiting multiple copies of activators or repressors.
This enables control of complex processes like:
Cell fate decisions, by co-activating or repressing multiple lineage-specific TFs.
Synthetic gene circuits, with logic gates constructed from epigenetically controlled nodes.
Synergistic enhancer activation, by targeting multiple distal enhancers converging on a common promoter.
Key points:
Multiple loci can be epigenetically edited in parallel.
Modular recruitment of distinct effectors via RNA or protein scaffolds.
Enables precise modeling and reprogramming of gene regulatory networks.
Technical Challenges
Efficiency and Persistence
Some modifications are transient unless reinforced. Methylation may not be maintained without DNMT1 activity during replication.Chromatin Accessibility
Pre-existing chromatin state affects targeting; closed chromatin may reduce dCas9 binding efficiency.Off-target Effects
Despite avoiding DSBs, nonspecific recruitment of effectors (e.g., global histone acetylation) can still perturb epigenomes.Allele Specificity
Targeting monoallelically (e.g., for imprinting disorders) is still an emerging capability.Multiplexing
Coordinating simultaneous editing of multiple loci is complex due to delivery constraints and effector crosstalk.
Emerging Developments
Split-effector systems: Effector activity only when two domains colocalize, improving specificity.
Optogenetic and chemical control: Light- or ligand-inducible systems for dynamic regulation.
CRISPRoff/CRISPRon: Systems for long-term gene silencing or activation via epigenetic marks without changing sequence.
Single-cell resolution: Integration with single-cell transcriptomics and epigenomics to analyze heterogeneity.
Modular and Multivalent Effector Architectures
Recent advances have focused on increasing the potency and modularity of epigenetic editors by enabling multivalent recruitment of effectors and combinatorial domain assembly.
SunTag system: A tandem array of GCN4 peptide epitopes is fused to dCas9, allowing the recruitment of multiple copies of a single-chain variable fragment (scFv)-effector fusion protein (e.g., scFv-p300 or scFv-VP64). This multiplicity amplifies the local enzymatic activity, increases histone mark deposition density, and enhances transcriptional output or repression depth.
MS2/PP7 scaffolding: Incorporates RNA aptamer stem-loops into the sgRNA scaffold (e.g., MS2 or PP7 loops), which bind to coat proteins fused to epigenetic effectors. This enables RNA-guided recruitment of multiple different effectors, allowing simultaneous activation and repression at distinct loci, or combinatorial control at a single locus.
Suntag + MS2 hybrid systems combine protein-based and RNA-based scaffolding to achieve orthogonal multimerization, enabling spatial colocalization of synergistic effectors (e.g., KRAB + DNMT3A, or p300 + TET1).
dCas9-Tethered Effector Arrays: Emerging designs include tandem fusion constructs (e.g., dCas9-KRAB-DNMT3A or dCas9-p300-VP64) or split-intein-mediated assembly, allowing delivery of very large editor proteins via compact systems (e.g., dual-AAV or mRNA constructs).
Key points:
SunTag and MS2/PP7 systems enhance effector density and modularity.
Combinatorial effectors enable precise chromatin remodeling profiles.
Split-intein and tandem fusions increase versatility of large fusion constructs.
Temporal and Spatial Control of Epigenetic Editors
Precise temporal and spatial control over epigenetic editing is essential for dissecting dynamic gene regulation and ensuring safety in therapeutic contexts. Several inducible and switchable systems are under development:
Optogenetic control: dCas9 or effector domains are fused to light-sensitive heterodimerization modules (e.g., CRY2-CIB1, PhyB-PIF). Upon light exposure (e.g., blue or red light), components heterodimerize, enabling precise, reversible, light-controlled recruitment of effectors to chromatin. Systems such as Light-Activated CRISPR Effectors (LACE) allow subcellular or time-specific regulation of gene expression.
Chemically inducible systems: Small molecule-induced heterodimerization domains (e.g., FKBP–FRB with rapamycin, ABI–PYL1 with abscisic acid) are used to bring together DNA-binding and effector domains conditionally. These systems can be finely tuned by ligand concentration and kinetics.
Proteolysis targeting: Incorporation of destabilization domains (DDs) or auxin-inducible degrons (AID) allows rapid degradation of epigenetic editors unless stabilized by specific ligands, permitting transient or oscillatory chromatin editing.
Split-dCas9 architectures: Functional dCas9 is split into two inactive fragments (N- and C-terminal domains), each fused to heterodimerizing modules. Only upon dimerization (light, ligand) is the full DNA-binding activity reconstituted, adding an additional safeguard layer.
Key points:
Light- and ligand-inducible systems offer spatiotemporal precision.
Split-dCas9 and degron tags enable conditional editing and reversibility.
These tools enable live-cell perturbation studies and therapeutic control.
Allele-Selective and Imprinting-Responsive Editors
New work is expanding the allele selectivity of epigenetic editors by leveraging endogenous epigenetic asymmetries (e.g., imprinting marks, methylation, chromatin state) or cis-acting sequence variants (SNPs, indels).
Polymorphism-guided targeting: Custom sgRNAs are designed to bind only one allele by exploiting SNPs that create or disrupt the PAM site or cause mismatches in the seed region. This allows differential binding to mutant vs. wild-type alleles.
Methylation-sensitive CRISPR systems: Development of methylation-sensitive dCas9 variants (e.g., Me-dCas9) that bind preferentially to methylated or unmethylated DNA based on PAM context or engineered DNA recognition modules. These can be paired with ChIP/ATAC-seq data for dynamic, cell-type-specific targeting.
Imprinting-specific regulation: Use of dCas9-KRAB or -TET1 to selectively repress or activate alleles within imprinted gene clusters (e.g., IGF2/H19, UBE3A/UBE3A-ATS). CRISPR-based targeting of long non-coding RNAs or antisense RNAs (e.g., Airn, Kcnq1ot1) is being explored to modulate allele-specific expression.
Base editing-enhanced specificity: Coupling epigenetic editors with adenine or cytosine base editors enables permanent creation of allele-specific PAM sites for conditional editing in only the desired cell population or allele.
Key points:
SNPs and methylation patterns enable allele-selective editing.
Methylation-sensitive Cas9 variants allow dynamic targeting of epigenetic states.
Imprinted loci can be selectively modulated without sequence alteration.
Epigenetic Recording and Synthetic Memory Systems
Synthetic biology is driving the development of epigenetic recorders—systems that encode cellular history into heritable chromatin states or molecular barcodes.
CRISPRoff / CRISPRon: dCas9 fused to KRAB-DNMT3A-L or TET1-p300 can install or erase durable epigenetic states at gene promoters without modifying the sequence. These marks can persist through cell division, enabling permanent silencing or activation with a single transient pulse.
Histone-based memory writers: dCas9 fusions to histone methyltransferases (e.g., PRC2 or SUV39H1) allow programming of H3K27me3 or H3K9me3 domains that recruit self-reinforcing feedback loops (reader–writer pairs like EED/EZH2 or HP1/SUV39H1), resulting in bistable or switch-like chromatin states.
Epigenomic barcoding: Tools such as Casilio (dCas9-PUF–based recruitment of barcoded RNA effectors) or CRISPRa/i libraries with embedded barcodes allow cell lineages or expression changes to be tracked over time at single-cell resolution using scRNA-seq + scATAC-seq.
Logic-based synthetic circuits: Modular use of dCas9-based repressors and activators with Boolean logic gates (e.g., AND, NOT) permits construction of programmable circuits for gene regulation that respond to combinations of input signals with memory storage via chromatin marks.
Key points:
CRISPRoff/on systems install long-term chromatin states without DNA edits.
Histone modifications enable bistable, heritable transcriptional states.
Barcoded epigenetic editors enable lineage tracing and logic-based gene control.
Clinical Translation and In Vivo Applications
Epigenetic editing is now entering preclinical and early clinical translation, with delivery and safety being the primary challenges. Emerging strategies include:
Non-viral delivery systems:
Lipid nanoparticles (LNPs) have been optimized for dCas9 mRNA or RNP delivery in vivo, enabling transient, localized chromatin editing (e.g., in the liver or CNS).
Polymeric nanoparticles, gold nanoclusters, or exosomes are being developed for tissue-specific targeting and improved biocompatibility.
Dual-vector AAV systems: Overcoming the payload limit of AAV (~4.7 kb) by splitting dCas9-effector constructs into two vectors reconstituted by intein splicing, trans-splicing ribozymes, or split-Cre systems.
Cell-type targeting via synthetic promoters or miRNA switches: Use of cell-specific transcriptional control elements or miRNA-targeted degradation sequences (e.g., miR-122 in hepatocytes) allows selective expression of epigenetic editors in defined cell populations.
Ex vivo engineering of therapeutically relevant cells:
CAR-T cells with epigenetically silenced exhaustion markers (e.g., PD-1).
iPSCs with stably repressed oncogenes or activated tissue-specific transcriptional programs.
HSPCs edited ex vivo for HbF reactivation via BCL11A enhancer silencing (a leading therapeutic application).
Key points:
LNPs and non-viral delivery platforms are enabling in vivo applications.
Dual-AAV systems and synthetic promoters enable cell-type specific targeting.
Ex vivo epigenome editing of therapeutic cell products is a leading application.
Conclusion
Epigenetic editing has emerged as a transformative paradigm in the field of gene regulation, offering a powerful alternative to traditional genome editing technologies. Rather than rewriting the genetic code through permanent sequence alterations or double strand breaks, epigenetic editing operates through the precise installation, removal, or modulation of chromatin marks—such as DNA methylation and histone modifications—at defined loci. This allows researchers to reprogram gene expression in a targeted, reversible, and non mutagenic manner, opening new avenues for basic research and therapeutic innovation.
Throughout this article, we've explored the mechanistic underpinnings of epigenetic regulation—DNA methylation, histone post translational modifications, and chromatin remodeling—and how these layers of control converge to shape transcriptional outcomes. Epigenetic editing leverages this regulatory machinery by combining sequence specific DNA binding platforms (such as dCas9, TALEs, or ZFPs) with catalytic effector domains (like DNMT3A, TET1, p300, KRAB, and EZH2). These fusions allow for locus specific recruitment of chromatin modifying activity to either silence, activate, or reprogram genes without damaging the genome, a key distinction from nuclease based CRISPR systems.
One of the most compelling features of epigenetic editing is its ability to modulate gene expression within the native chromatin environment, preserving the three dimensional architecture, regulatory element interactions, and context dependent dynamics of the genome. This native context targeting is crucial for studying developmental gene programs, non coding regulatory regions, and dosage sensitive loci that cannot be accurately modeled by sequence deletion or exogenous overexpression. It also enables allele specific targeting, a vital requirement for imprinting disorders and dominant negative mutations, where selectively modulating one allele can restore normal function without collateral effects.
From a translational perspective, the clinical potential of epigenetic editing is rapidly advancing, supported by innovations in delivery platforms (e.g., LNPs, AAV, RNPs), inducible control systems (e.g., optogenetic and ligand responsive switches), and multivalent architectures (e.g., SunTag and MS2/PP7 scaffolds). These advances improve cell type specificity, editing efficiency, and safety—key factors in therapeutic applications. Use cases such as in vivo reactivation of fetal hemoglobin for β thalassemia, silencing of latent HIV reservoirs, modulation of immune checkpoint expression, and engineering of iPSCs or CAR T cells underscore the breadth of potential for both ex vivo and systemic interventions.
Epigenetic editing also offers unique capabilities that extend beyond therapy, enabling the creation of synthetic memory systems, lineage recorders, and logic based gene circuits. These applications blur the line between biology and computation, allowing scientists to encode information directly into the epigenome and build programmable cells that respond to environmental or endogenous signals with heritable, state dependent behaviors. These tools hold enormous promise for synthetic biology, regenerative medicine, and systems level understanding of complex gene networks.
Importantly, epigenetic editing invites a broader conceptual shift in how we define genetic function. The historical view of the genome as a static blueprint is giving way to a more nuanced understanding that gene activity is determined not only by sequence, but by the dynamic and context sensitive epigenetic landscape that overlays it. This realization underscores why precise, context aware, and reversible control of gene expression is essential—not only for unraveling fundamental biology, but for engineering cells and tissues in a way that is safe, flexible, and predictable.
In conclusion, epigenetic editing is not merely a refinement of existing genome engineering tools—it represents a fundamentally different and in many ways more sophisticated modality. Its ability to control gene expression with high specificity, reversibility, and heritability—without introducing mutations—positions it as a cornerstone technology for the next generation of genomic medicine, functional genomics, and synthetic biology. As the field continues to mature, epigenetic editing is poised to revolutionize our ability to interrogate, interpret, and manipulate the genome at the level of regulation rather than sequence—ushering in a new era of precision control over cellular identity and function.
Glossary of Terms – Epigenetic Editing
Epigenetic
Refers to changes in gene activity that do not involve altering the DNA sequence itself.DNA Methylation
The addition of a chemical tag (methyl group) to DNA that can turn genes off.Histone
Proteins that DNA wraps around to form chromatin; they help control whether genes are turned on or off.Chromatin
The complex of DNA and proteins (mainly histones) that makes up chromosomes inside the nucleus.Post-Translational Modifications (PTMs)
Chemical changes made to proteins after they are produced, which affect how they function—especially on histones.Acetylation
A chemical modification that typically makes DNA more open and genes more active.Methylation (of histones)
A modification that can either turn genes on or off depending on where it happens.Demethylation
The removal of methyl tags, often used to turn genes back on.Nucleosome
A unit of DNA wrapped around histone proteins, like thread wrapped around a spool.Gene Expression
The process of turning genetic information into proteins or other molecules the cell needs.Silencing
Turning a gene off so it doesn't produce anything.dCas9 (dead Cas9)
A version of the CRISPR protein Cas9 that can bind DNA but cannot cut it.TALEs / Zinc Finger Proteins (ZFPs)
Custom proteins that can be engineered to bind to specific DNA sequences.Guide RNA (gRNA or sgRNA)
A short piece of RNA that guides dCas9 to a specific DNA location.KRAB
A protein domain that can turn genes off by forming repressive chromatin.p300
An enzyme that turns genes on by adding acetyl tags to histones.TET1
An enzyme that removes methylation marks from DNA, helping to reactivate genes.DNMT3A
An enzyme that adds methylation marks to DNA, which usually turns genes off.EZH2 / PRC2
A complex that adds repressive marks to histones, often silencing genes.H3K27ac / H3K27me3
Chemical tags on histone H3 that indicate active (ac) or repressed (me3) genes.5mC (5-methylcytosine)
A methylated form of the DNA base cytosine, associated with gene silencing.CpG Island
DNA regions rich in CG sequences, often found in gene promoters and usually unmethylated in active genes.Protospacer Adjacent Motif (PAM)
A short DNA sequence next to the target site that dCas9 needs to bind DNA.Chromatin Remodelers
Proteins that use energy to move or change nucleosomes to make DNA more or less accessible.Lipid Nanoparticles (LNPs)
Tiny fat-based particles used to deliver genetic material or proteins into cells.AAV / Lentivirus / Adenovirus
Different types of viruses used to deliver DNA into cells in a lab or therapy.Split-Intein
A protein trick where two halves of a protein are delivered separately and then stitched together inside the cell.MS2 / PP7 System
An RNA-based system that helps bring more protein effectors to specific DNA sites.SunTag
A protein-based system that boosts the number of effectors recruited to a single DNA location.CRISPRoff / CRISPRon
Systems that can turn genes off or on using epigenetic changes, without cutting DNA.RNP (Ribonucleoprotein)
A complex of a protein (like dCas9) and RNA (like a guide) delivered directly into cells.Heterochromatin / Euchromatin
Heterochromatin is tightly packed and inactive; euchromatin is open and active.Imprinting
A genetic phenomenon where only one of the two gene copies (from mother or father) is active.Allele
A different version of a gene—one inherited from each parent.Single-Cell Resolution
The ability to study gene activity in individual cells, rather than averages across many cells.Lineage Tracing
A method for tracking how a cell and its descendants change over time.Synthetic Biology
A field that engineers biological systems, like cells, to perform specific tasks or logic functions.
Logic Gates (AND, NOT, etc.)
Rules used in synthetic biology to control when genes are turned on or off, based on specific inputs.


🧬 Support Independent Biotech Journalism 🧬
At BiotechnologyReviews.com, we’re committed to delivering in-depth, science-driven content that explores the cutting edge of genetics, molecular biology, and therapeutic innovation — all free and accessible to readers worldwide.
If you value high-quality, expertly researched articles on breakthroughs like epigenetic editing, gene therapy, and CRISPR-based technologies, we invite you to support our work.
Your pledge helps us:
Publish rigorous articles free from clickbait and hype
Cover underreported topics shaping the future of medicine and biotech
Keep our content independent, ad-light, and accessible to all
🔗 Make a difference — pledge your support today at https://lnkd.in/dDYUMY5g
Together, we can empower science-literate conversation and drive forward a more informed biotech future.