Multispecific Antibodies (msAbs), A Complete Overview
Scientific Digital Marketing, Synthetic Biology, Nucleic Acid Therapeutics and Antibody Engineering, Biotech Writer
Multispecific antibodies (msAbs) represent a groundbreaking evolution in antibody engineering, offering the ability to engage multiple targets simultaneously. Unlike traditional monoclonal antibodies (mAbs), which bind to a single epitope on a single antigen, msAbs are designed to recognize and interact with multiple epitopes across different antigens or even distinct cell types. This multi-targeting capability opens new avenues for therapeutic applications, particularly in oncology, autoimmune diseases, infectious diseases, and neurodegenerative disorders. By orchestrating complex biological interactions, msAbs enhance treatment specificity, reduce off-target toxicity, and improve clinical outcomes.
The development of msAbs has been driven by significant advancements in genetic engineering, protein expression systems, and molecular biology. Since their initial conception in the 1980s, scientists have refined production techniques to overcome early challenges such as poor chain pairing and stability issues. Landmark breakthroughs, including the FDA approvals of Catumaxomab in 2009 and Blinatumomab in 2014, demonstrated the clinical viability of msAbs, paving the way for next-generation formats with improved manufacturability and efficacy. Today, cutting-edge platforms such as CrossMAb, DuoBody, and Knob-into-Hole technology have further expanded the potential of msAbs in immunotherapy and beyond.
Compared to traditional monoclonal and polyclonal antibodies, msAbs offer numerous advantages. They can bind multiple tumor antigens, reducing the likelihood of resistance mechanisms such as antigen escape. They also enable immune cell redirection, where cytotoxic T-cells or natural killer (NK) cells are guided directly to cancer cells, increasing the efficiency of tumor eradication. Moreover, by simultaneously blocking multiple signaling pathways or immune checkpoints, msAbs offer a more robust and durable therapeutic effect. These advantages make msAbs particularly appealing for personalized medicine, where treatments can be tailored to an individual’s specific disease profile.
As the field of multispecific antibody engineering continues to evolve, researchers are exploring novel formats such as trispecific and tetraspecific antibodies, antibody-drug conjugates (ADCs), and nanobody-based platforms. Additionally, AI-driven antibody discovery, CRISPR-mediated optimization, and synthetic biology approaches are accelerating the design and development of next-generation msAbs. This article will provide an in-depth exploration of multispecific antibodies, covering their classification, structural engineering, production methodologies, mechanisms of action, and the challenges that must be overcome to fully realize their therapeutic potential.
Key Article Content Topics:
Definition and Distinction from Conventional Monoclonal Antibodies
Historical Development and Scientific Breakthroughs
Advantages of Multispecific Antibodies
Classification of Multispecific Antibodies
Molecular Design and Structural Engineering
Mechanisms of Action in Disease Treatment
Production and Expression Systems
Challenges and Limitations
Next-Generation Multispecific Antibody Technologies
Classification: bispecific, trispecific, and beyond
Definition and Distinction from Conventional Monoclonal Antibodies (mAbs)
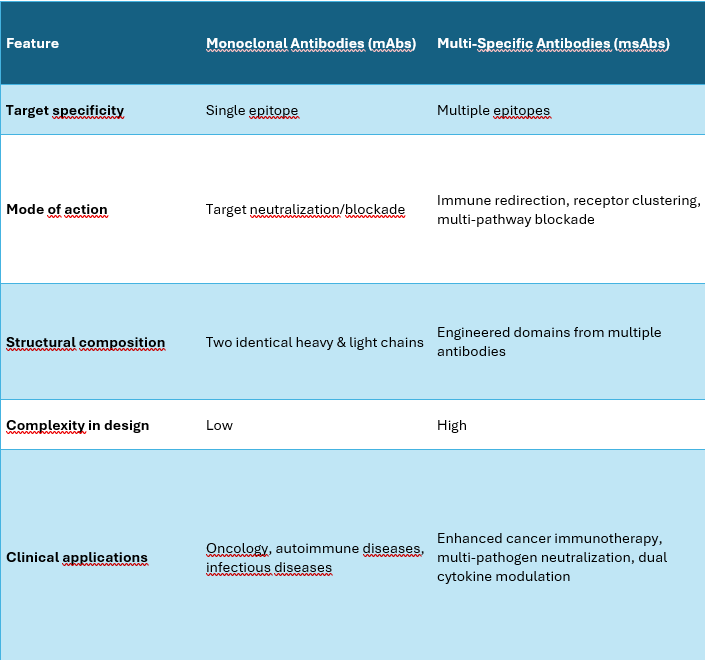
Monoclonal Antibodies (mAbs) vs. Multispecific Antibodies (msAbs)
Monoclonal antibodies (mAbs) are highly specific immunoglobulins (IgGs) that recognize and bind to a single epitope on a single target antigen. They are widely used in therapeutics, diagnostics, and research due to their high affinity, specificity, and predictable pharmacokinetics.
In contrast, multispecific antibodies (msAbs) are engineered to bind to two or more distinct epitopes, which may be:
On the same antigen (e.g., enhancing binding efficiency)
On different antigens (e.g., linking tumor cells to immune cells)
On different cells (e.g., engaging immune effectors)
This multi-targeting ability enhances therapeutic efficacy by:
Increasing specificity while reducing off-target effects.
Orchestrating complex biological interactions, such as redirecting immune cells to tumors or blocking multiple disease pathways simultaneously.
Improving pharmacokinetic (PK) and pharmacodynamic (PD) properties by controlling binding avidity, retention time, and antigen internalization rates.
Structural Differences
Historical Development and Scientific Breakthroughs
Multispecific antibodies were first conceived in the 1980s as a way to overcome the limitations of conventional mAbs. Over time, advancements in genetic engineering, protein expression, and molecular biology have led to the development of highly stable, manufacturable, and clinically effective msAbs.
Key Milestones in Multispecific Antibody Development
1983 – First Bispecific Antibodies (BsAbs) via Hybrid Hybridomas Köhler and Milstein’s hybridoma technology (which led to monoclonal antibodies) was adapted to create quadromas—hybridomas fusing two different B-cell clones, producing bispecific antibodies. Limitation: Poor chain pairing and instability.
1990s – Chemical Crosslinking and Recombinant Engineering Chemical conjugation of two mAbs created bispecific molecules, but these had low stability and high aggregation. Recombinant engineering improved chain pairing, flexibility, and pharmacokinetics.
2009 – First FDA-Approved Bispecific Antibody (Catumaxomab) Targets EpCAM/CD3 for peritoneal carcinomatosis. Demonstrated the potential of bispecific antibodies but had limitations in manufacturability.
2014 – FDA Approval of Blinatumomab (CD19/CD3) First-in-class bispecific T-cell engager (BiTE), transforming cancer immunotherapy. Demonstrated superior efficacy in B-cell acute lymphoblastic leukemia (B-ALL). Kickstarted the clinical adoption of multispecific platforms.
2016–Present – Evolution of Advanced msAbs CrossMAb (Roche), DuoBody (Genmab), and Knob-into-Hole (Amgen) improved manufacturability and stability. FDA/EMA approvals of multispecific drugs for hematological malignancies and solid tumors. Advancements in AI-driven antibody discovery and next-gen formats (tri-/tetra-specific Abs, antibody-fusion proteins, and nanobody-based msAbs).
Advantages of Multispecific Antibodies Over Traditional Monoclonal and Polyclonal Antibodies
Improved Targeting Efficiency
Monoclonal antibodies bind a single antigen, which may lead to resistance mechanisms (e.g., antigen escape).
Multispecific antibodies can: Bind multiple tumor antigens simultaneously, reducing the chance of antigen escape. Engage multiple immune pathways, improving immune response efficiency.
Enhanced Therapeutic Mechanisms
Immune Cell Redirection: Bispecific T-cell engagers (BiTEs) bind both CD3 (T-cells) and tumor antigens, directing T-cell cytotoxicity.
Receptor Clustering and Signaling Enhancement: Some msAbs bring together receptors (e.g., HER2/HER3) to induce enhanced signaling inhibition.
Checkpoint Blockade Combination: Multispecific checkpoint inhibitors can target PD-1, CTLA-4, LAG-3 in one molecule, avoiding combination therapy limitations.
Reduced Systemic Toxicity
Instead of administering two separate monoclonal antibodies, a multispecific antibody localizes the activity to target cells, reducing systemic immune activation and off-target toxicity.
Lower Manufacturing and Development Costs
Traditional combination therapies require separate development, testing, and regulatory approval for each antibody.
A single multispecific antibody can replace multiple drugs, simplifying clinical development, storage, and distribution.
Greater Potential for Personalized Medicine
Multispecific antibodies can be engineered for specific patient profiles, optimizing immune engagement and reducing resistance.
Classification of Multispecific Antibodies: Bispecific, Trispecific, and Beyond
Multispecific antibodies are categorized based on the number of target epitopes they engage.
Most common class of multispecifics.
Bind two different targets.
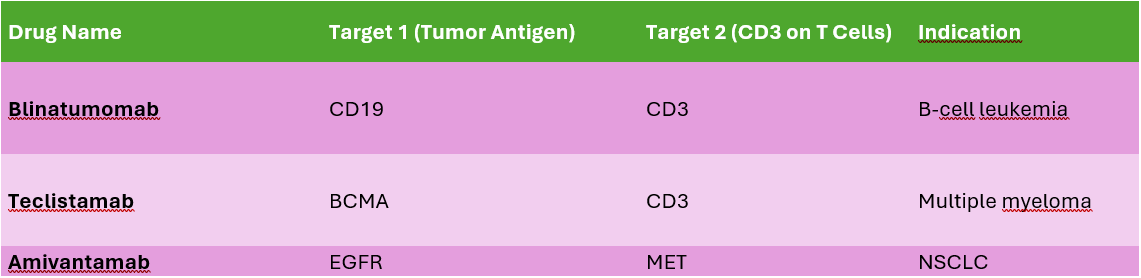
Examples: Blinatumomab (CD19/CD3) – Redirects T-cells to B-cell tumors. Emicizumab (FIXa/FX) – Mimics factor VIII in hemophilia.
🔬
Fragment-based (scFv, BiTEs, DARTs) – Smaller, more flexible.
Full-length IgG-based (CrossMAb, DuoBody, Knob-into-Hole) – Enhanced stability.
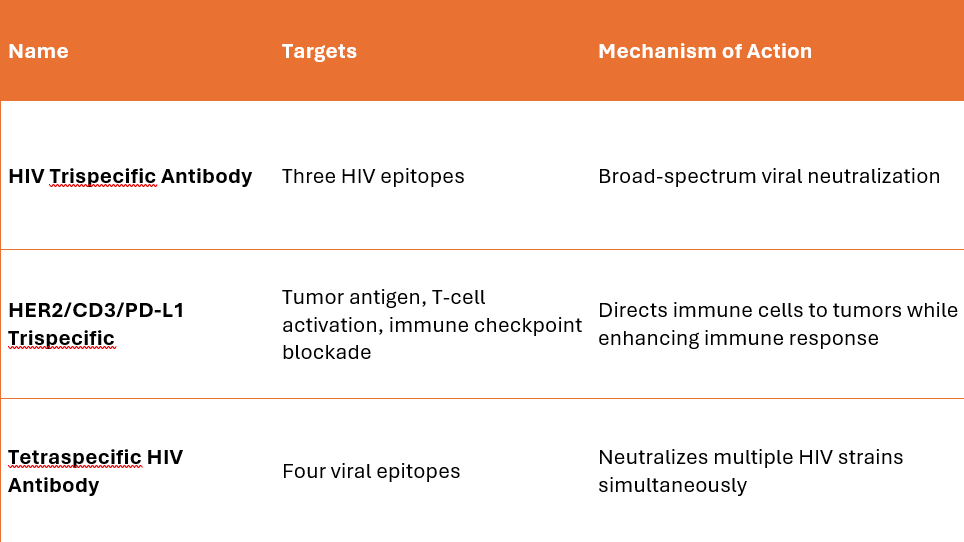
Trispecific Antibodies
Bind three different targets simultaneously.
Examples: Anti-HIV trispecific antibodies – Target multiple viral epitopes. Cancer trispecifics (e.g., HER2/CD3/PD-L1) – Redirect immune cells while blocking tumor immune evasion.
🔬 Mechanisms:
Triple immune cell engagement (e.g., T-cell, NK-cell, tumor cell).
Multi-pathway blockade in cancer therapy.
Tetraspecific and Beyond
Engineered to target four or more epitopes.
Example: HIV tetraspecific Abs neutralizing multiple viral strains.
🔬 Challenges: Structural complexity. Ensuring correct heavy-light chain pairing.
Multispecific antibodies represent a paradigm shift in therapeutic antibody engineering. They offer unmatched versatility, higher efficacy, and lower systemic toxicity compared to conventional mAbs. Recent technological advancements have enabled clinically viable bispecific, trispecific, and even tetraspecific antibodies, paving the way for next-generation immunotherapies, personalized medicine, and novel disease-targeting strategies.
Molecular Design and Structural Engineering
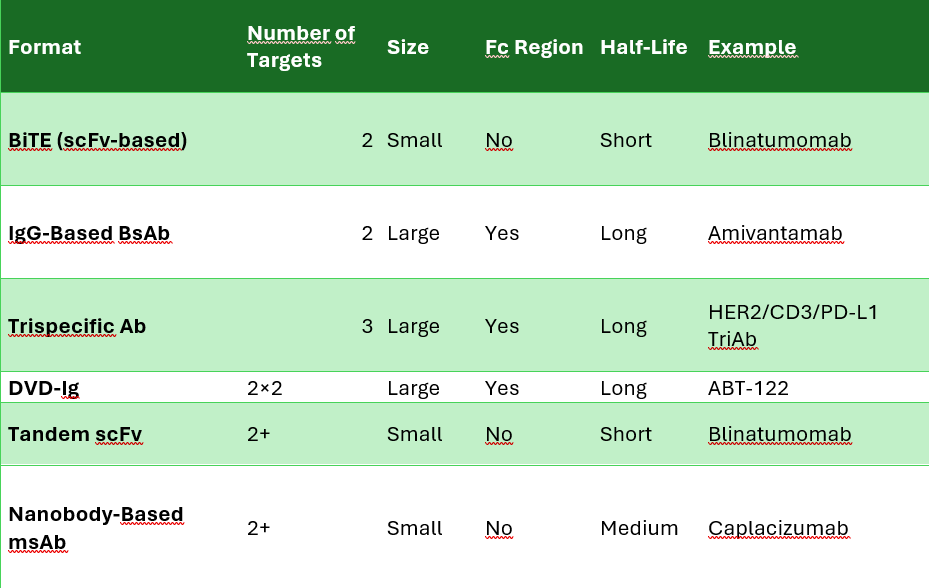
Types of multispecific antibodies Bispecific antibodies (BsAbs) Trispecific and tetraspecific antibodies Dual-variable domain antibodies (DVD-Ig) Tandem single-chain variable fragments (scFv) Nanobody-based multispecific formats
Molecular Design and Structural Engineering of Multispecific Antibodies
The molecular design of multispecific antibodies (msAbs) is highly sophisticated, requiring precise engineering of binding sites, stability optimization, and functional efficacy. Below is a deep dive into various types of multispecific antibodies and their structural innovations.
Types of Multispecific Antibodies
Bispecific Antibodies (BsAbs)
Bispecific antibodies (BsAbs)
T-cell redirection (e.g., CD3-TAA bispecifics for cancer therapy).
Dual blockade of signaling pathways (e.g., HER2/HER3 inhibition).
Enhanced receptor clustering for synergistic activation or inhibition.
Structural Formats of BsAbs
BiTEs (Bispecific T-cell Engagers) – Two single-chain variable fragments (scFvs) linked by a flexible peptide. Example: Blinatumomab (CD19/CD3).
DARTs (Dual Affinity Retargeting Antibodies) – Two scFvs engineered for improved stability and avidity.
B. IgG-Based BsAbs
Knob-into-Hole Technology – A modified Fc domain allows selective heavy chain pairing. Example: Amivantamab (EGFR/MET).
CrossMab – A domain-exchanged format improving chain pairing. Example: RG7716 (Ang-2/VEGF).
DuoBody – Genmab's platform using controlled Fab arm exchange.
Trispecific and Tetraspecific Antibodies
Multispecific antibodies beyond bispecifics target three or more antigens, allowing:
Triple immune cell engagement (e.g., CD3, CD28, and tumor antigen).
Combination checkpoint blockade (e.g., PD-1, LAG-3, and TIM-3 inhibition).
Multi-pathogen neutralization (e.g., HIV or COVID-19 therapies).
Examples of Trispecific and Tetraspecific Antibodies
🔬 Engineering Challenges:
Complex chain pairing and stability issues.
Increased immunogenicity risk due to novel structures.
Ensuring correct stoichiometry for optimal function.
Dual-Variable Domain Antibodies (DVD-Ig)
DVD-Ig antibodies are an innovative format featuring two independent antigen-binding sites per Fab arm, allowing quadruple specificity (2×2 targets).
Structural Features
Two sets of variable regions (VH-VL pairs) are stacked, forming a dual-binding interface.
Top layer engages one target, while bottom layer engages another.
Retains Fc-mediated functions.
Advantages
Enhanced avidity due to multivalent binding.
Dual cytokine inhibition (e.g., IL-17/IL-23 blockade in autoimmune diseases).
Greater functional flexibility in cancer immunotherapy.
Example
ABT-122 (DVD-Ig targeting TNF-α and IL-17 for rheumatoid arthritis).
Tandem Single-Chain Variable Fragments (scFv)
Tandem scFvs consist of multiple antigen-binding domains fused in a linear configuration, enabling flexible multi-target engagement.
Structural Features
Composed of two or more scFv domains linked by short peptide linkers.
Completely lacks an Fc region (non-IgG-like).
Small size enables deep tissue penetration.
Advantages
High flexibility and modularity.
Efficient bispecific or trispecific targeting.
Lower manufacturing complexity compared to full-length IgG-based msAbs.
Example
Blinatumomab (CD19/CD3 BiTE) – A tandem scFv format that brings T-cells into proximity with tumor cells.
🔬 Challenges:
Short half-life, requiring continuous infusion.
Higher aggregation risk due to scFv instability.
Nanobody-Based Multispecific Formats
Nanobody-based multispecific antibodies leverage single-domain antibodies (sdAbs) derived from camelid heavy-chain-only antibodies.
Structural Features
Composed of one or more nanobody domains (15 kDa vs. 150 kDa for IgG).
Highly stable, resistant to denaturation, and can penetrate challenging tissues.
Can be engineered into bispecific, trispecific, or multispecific constructs.
Advantages
Superior tissue penetration, particularly for brain and solid tumors.
Can be formatted as bispecific immune cell engagers.
Easier and cheaper to manufacture.
Example
Caplacizumab – A bispecific nanobody targeting vWF in thrombotic thrombocytopenic purpura (TTP).
Comparison of Multispecific Antibody Formats
Multispecific antibody engineering is transforming immunotherapy, oncology, and infectious disease treatments. Each format has unique advantages tailored to different clinical applications:
Bispecific antibodies dominate T-cell engagement and dual blockade strategies.
Trispecific and tetraspecific antibodies enable more complex immune modulations.
DVD-Ig and tandem scFv offer greater functional diversity.
Nanobody-based formats provide high stability, deep penetration, and novel delivery advantages.
🔬 Future Directions
AI-driven antibody optimization to design novel multispecific architectures.
Fusion with cell therapies (CAR-T, NK cells) for enhanced efficacy.
Multispecific ADCs (Antibody-Drug Conjugates) for targeted payload delivery.
Design Approaches Knob-into-hole technology CrossMab, DuoBody, and other scaffold designs Fusion proteins and chemical crosslinking Heavy and light chain pairing challenges Glycoengineering and post-translational modifications.
Design Approaches in Multispecific Antibody Engineering
The structural complexity of multispecific antibodies (msAbs) requires precise engineering to ensure correct heavy-light chain pairing, stability, manufacturability, and efficacy. Below is an in-depth breakdown of the key molecular design approaches used in engineering bispecific, trispecific, and multispecific antibodies.
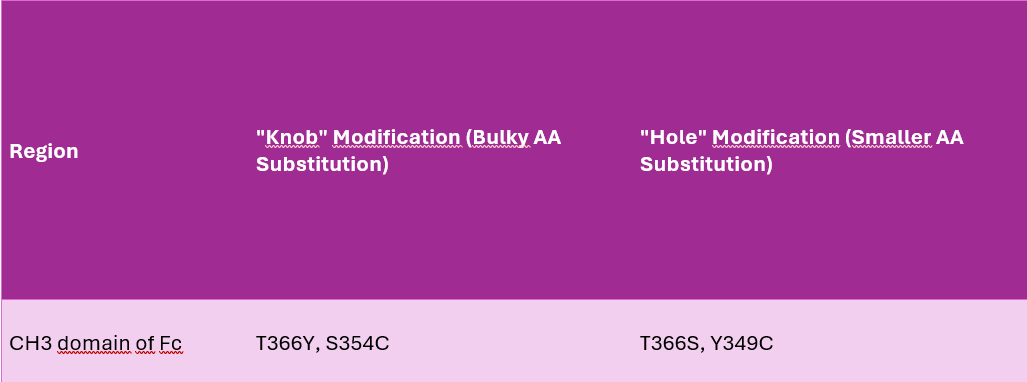
Knob-into-Hole Technology
What is Knob-into-Hole (KiH)?
Knob-into-Hole (KiH) is a Fc-engineering approach that ensures correct heterodimerization of two different heavy chains in bispecific antibodies.
It was developed to overcome heavy chain mispairing in IgG-based bispecific antibodies.
Molecular Design
One heavy chain is engineered to have a “knob” (a bulky amino acid substitution).
The other heavy chain has a complementary “hole” (smaller amino acids).
This promotes preferential pairing of the correct heavy chains while minimizing the formation of unwanted homodimers.
Key Amino Acid Modifications
Advantages
✅ Increased heterodimer formation (~90% pairing efficiency).
✅ Compatible with Fc-based IgG scaffolds, retaining Fc-mediated functions (e.g., ADCC, CDC).
✅ Scalable for large-scale manufacturing in CHO cells.
Limitations
❌ Some degree of homodimer formation still occurs (~5-10%).
❌ Requires additional mutations to fine-tune stability.
Examples
Amivantamab (EGFR/MET bispecific antibody) – Uses KiH Fc-engineering for therapeutic efficacy in lung cancer.
Zanidatamab (HER2/HER2 bispecific antibody) – Uses Fc-based bispecific scaffolds.
CrossMAb, DuoBody, and Other Scaffold Designs
Several scaffold designs have been developed to ensure correct light-chain pairing while retaining Fc-mediated effector functions.
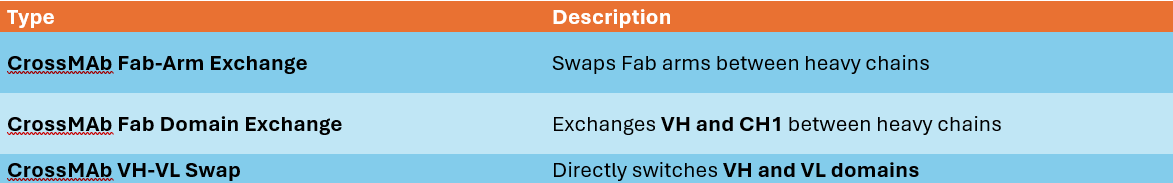
A. CrossMAb Technology
CrossMAb (Crossed Monoclonal Antibodies) is an approach developed by Roche to prevent incorrect light chain pairing.
Structural Design
Heavy chains are standard, but the Fab regions undergo domain exchange: Either VH-CH1 or VL-CL is swapped between chains. This ensures that each heavy chain pairs with the correct light chain.
CrossMAb Variants
Advantages
✅ Eliminates mispaired light chains, increasing functional antibody yield.
✅ Retains Fc-mediated functions and has long serum half-life.
Example
Faricimab (VEGF-A/Ang-2 bispecific CrossMAb) – Used for age-related macular degeneration (AMD).
DuoBody Technology
DuoBody technology, developed by Genmab, utilizes Fab-arm exchange to create bispecific antibodies.
How DuoBody Works
Two different monoclonal IgG antibodies are expressed separately.
Under mild reducing conditions, the Fab arms dissociate.
Upon oxidation, Fab arms randomly reassemble, forming a bispecific antibody.
Advantages
✅ High efficiency (>90% correct bispecific formation).
✅ Fc retains full functionality (ADCC, CDC).
✅ Compatible with standard IgG production pipelines.
Examples
Epcoritamab (CD20/CD3 bispecific antibody) – A DuoBody engaging T cells to kill B-cell lymphomas.
Fusion Proteins and Chemical Crosslinking
Some multispecific antibodies use direct fusion of antibody fragments or chemical conjugation methods.
Fusion Proteins
Fusion proteins are engineered by genetically fusing multiple antigen-binding domains into a single polypeptide.
Examples of Fusion Protein-Based Multispecific Antibodies
Chemical Crosslinking
Early bispecific antibodies were produced by chemically crosslinking two different monoclonal antibodies using:
Thiol-reactive linkers (e.g., maleimide-based conjugation).
Heterobifunctional crosslinkers.
❌ Limitations of Chemical Crosslinking
High heterogeneity.
Low stability.
Not scalable for large-scale production.
🔬 Thus, modern multispecific antibodies rely on genetic engineering rather than chemical conjugation.
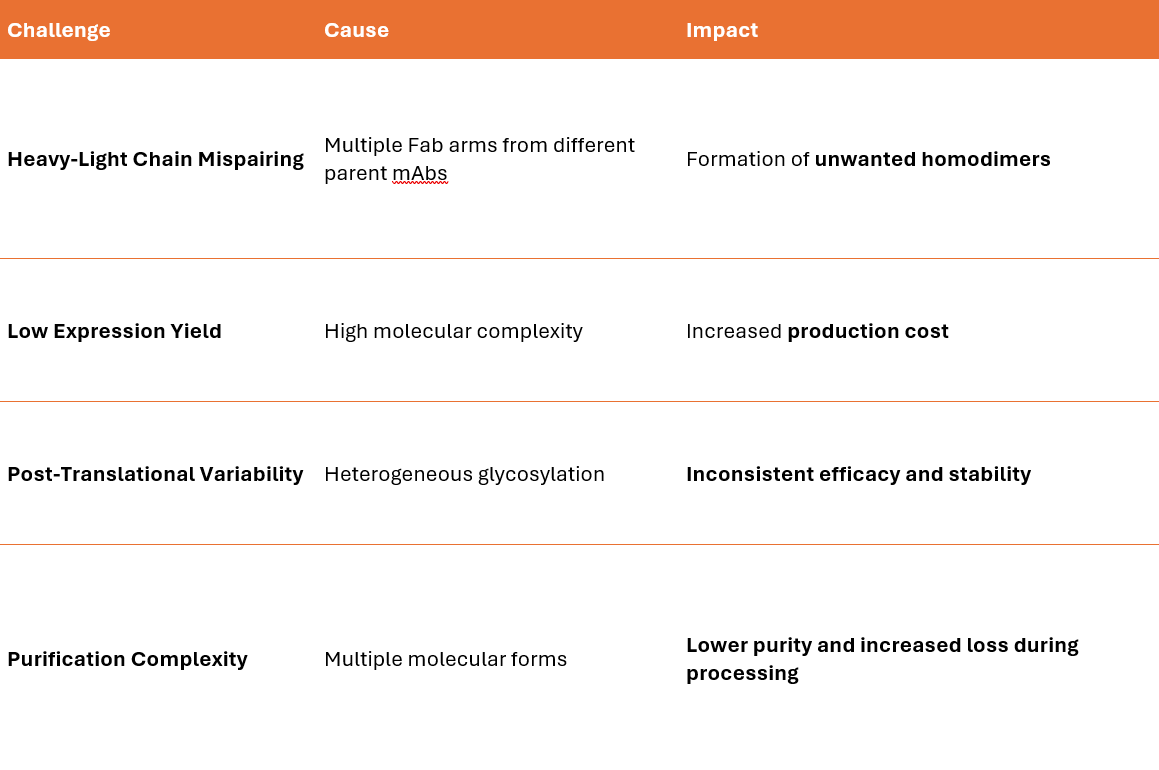
Heavy and Light Chain Pairing Challenges
One of the biggest obstacles in multispecific antibody engineering is correct heavy and light chain pairing.
Key Challenges
Light Chain Mispairing – In IgG-based bispecifics, a single light chain may incorrectly pair with the wrong heavy chain.
Heavy Chain Homodimerization – Instead of forming a heterodimer, two identical heavy chains may pair.
Solutions
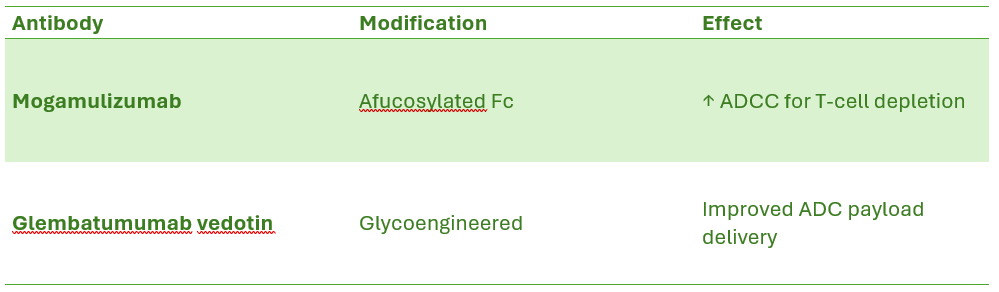
Glycoengineering and Post-Translational Modifications
Glycosylation plays a critical role in stability, immunogenicity, and effector function of multispecific antibodies.
A. Glycoengineering Strategies
Afucosylation – Removing fucose from Fc N-glycans enhances ADCC.
Sialylation – Adding sialic acids reduces FcγR binding, improving anti-inflammatory effects.
Site-Specific Glycan Engineering – Modifying glycosylation sites improves stability and solubility.
B. Other Post-Translational Modifications
Disulfide Engineering – Optimizing disulfide bridges enhances stability.
PEGylation – Attaching polyethylene glycol (PEG) extends half-life.
Examples of Glycoengineered Multispecific Antibodies
Multispecific antibody engineering requires advanced molecular strategies to overcome misfolding, chain mispairing, and stability challenges. Knob-into-Hole, CrossMAb, DuoBody, and glycoengineering are among the most effective solutions driving next-generation immunotherapy.
Mechanisms of Action
· Bridging two targets (e.g., tumor and immune cells)
· Enhanced receptor clustering and activation
· Blocking multiple signaling pathways
· Tumor microenvironment modulation
Mechanisms of Action of Multispecific Antibodies (msAbs)
Multispecific antibodies (msAbs) operate through highly sophisticated mechanisms of action that leverage their ability to bind multiple targets simultaneously. These mechanisms include:
Bridging two targets (e.g., tumor and immune cells)
Enhanced receptor clustering and activation
Blocking multiple signaling pathways
Tumor microenvironment modulation
Each of these mechanisms is designed to maximize therapeutic efficacy while reducing off-target toxicity. Below is an in-depth breakdown of these mechanisms.
Bridging Two Targets (e.g., Tumor and Immune Cells)
Concept:
Multispecific antibodies can act as a biological bridge to force an interaction between two different cell types or molecular targets. The most prominent application of this mechanism is T-cell redirection, where msAbs bring cytotoxic T cells into proximity with tumor cells.
Key Example: T-cell Engagers (TCEs)
Bispecific T-cell Engagers (BiTEs) and Trispecific T-cell Engagers (TriTEs) are prime examples of msAbs designed to bridge CD3 on T cells with tumor-associated antigens (TAAs).
Molecular Mechanism:
The msAb binds CD3 (T-cell receptor complex) on cytotoxic T lymphocytes (CTLs).
The other arm binds a tumor antigen (e.g., CD19, HER2, EGFR, BCMA).
This physically brings T cells and tumor cells into close proximity, bypassing the requirement for antigen presentation via MHC.
T cells become artificially activated, leading to: Granzyme B and perforin release → Tumor cell lysis. Cytokine secretion (IL-2, IFN-γ, TNF-α) → Enhanced immune response.
Examples of T-cell Engagers
Advantages
✅ Bypasses tumor immune evasion by directly engaging T cells.
✅ Does not require MHC presentation, overcoming immune evasion tactics.
✅ Highly potent, even at low concentrations.
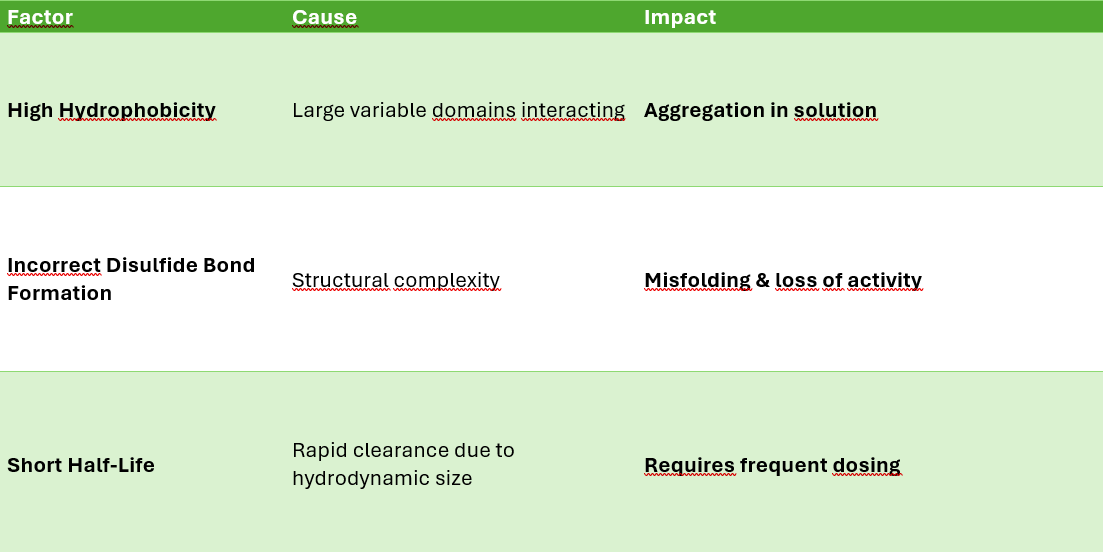
Challenges
❌ Risk of cytokine release syndrome (CRS) due to massive T-cell activation.
❌ Short half-life (e.g., BiTEs require continuous infusion).
🔬 Solutions:
Fc engineering to increase half-life.
Dual-checkpoint blockade (e.g., PD-1/CD3 bispecifics) to fine-tune immune activation.
Enhanced Receptor Clustering and Activation
Concept:
Some multispecific antibodies enhance receptor activation by bringing together multiple receptors into clusters, mimicking natural ligand-receptor interactions.
Key Example: HER2/HER3 Co-Targeting
HER2 and HER3 are heterodimerizing partners in many cancers.
Trastuzumab (HER2 mAb) alone does not completely inhibit HER2 signaling.
A bispecific HER2/HER3 antibody can force receptor clustering, leading to internalization and degradation.
Molecular Mechanism
The msAb binds two receptors (e.g., HER2 and HER3).
This induces dimerization or clustering, leading to: Increased ligand-independent activation (if an agonist). Receptor degradation (if an antagonist).
Enhances therapeutic response by blocking redundant signaling pathways.
Examples of Receptor Clustering-Based Antibodies
Advantages
✅ Higher efficacy than single-target antibodies.
✅ Prevents compensatory signaling, reducing drug resistance.
✅ Induces receptor degradation, providing long-term suppression.
Challenges
❌ Some receptors (e.g., PD-1, CTLA-4) need precise clustering to avoid overactivation.
❌ Potential for paradoxical activation, requiring careful structural optimization.
🔬 Solution:
AI-driven protein engineering to fine-tune epitope spacing.
Blocking Multiple Signaling Pathways
Concept:
Many diseases, especially cancer and autoimmune disorders, involve multiple redundant signaling pathways. A multispecific antibody can simultaneously block two or more of these pathways, preventing compensatory resistance.
Key Example: Dual Cytokine Blockade in Autoimmune Diseases
IL-17 and IL-23 are key inflammatory cytokines in psoriasis and rheumatoid arthritis.
Blocking both cytokines improves efficacy compared to single inhibitors.
Molecular Mechanism
msAb binds IL-17A and IL-23, preventing them from activating their respective receptors.
This results in reduced inflammatory cytokine secretion.
Lower T-cell activation, reducing disease progression.
Advantages
✅ Higher efficacy than single-target blockade.
✅ Prevents compensatory resistance mechanisms.
Challenges
❌ Increased immunosuppression risk.
❌ Potential for severe infections due to broad cytokine blockade.
🔬 Solution: Engineering conditional activation (e.g., tumor microenvironment-dependent binding).
Tumor Microenvironment Modulation
Concept:
The tumor microenvironment (TME) is often immunosuppressive, preventing immune cells from effectively killing cancer cells. Multispecific antibodies can reprogram the TME by:
Blocking immune checkpoints (e.g., PD-1, CTLA-4, LAG-3).
Recruiting immune effector cells (e.g., NK cells, macrophages).
Inducing pro-inflammatory cytokine secretion.
Key Example: PD-L1/CD137 Bispecifics
PD-L1 inhibits T-cell activation.
CD137 (4-1BB) is a costimulatory receptor.
A PD-L1/CD137 bispecific antibody can simultaneously block immune suppression and activate T cells.
Molecular Mechanism
The msAb binds PD-L1, preventing T-cell exhaustion.
Simultaneously, it binds CD137, providing a costimulatory survival signal to T cells.
This restores T-cell function, leading to tumor rejection.
Advantages
✅ Turns “cold” tumors into “hot” tumors.
✅ Enhances immune memory, preventing tumor recurrence.
Challenges
❌ Higher risk of autoimmunity.
❌ Potential for severe immune-related adverse events (irAEs).
🔬 Solution: Engineering conditional activation domains to reduce toxicity.
Multispecific antibodies leverage cutting-edge molecular design to:
✅ Redirect immune cells against tumors.
✅ Cluster receptors for enhanced therapeutic effects.
✅ Block multiple disease pathways.
✅ Reprogram the tumor microenvironment for long-term efficacy.
Structural Optimizations for Each Mechanism of Action in Multispecific Antibodies (msAbs)
To enhance the efficacy, stability, and specificity of multispecific antibodies (msAbs), various structural optimizations have been developed. Each mechanism of action—T-cell redirection, receptor clustering, pathway inhibition, and tumor microenvironment (TME) modulation—requires different structural engineering approaches.
Structural Optimization for Bridging Two Targets (e.g., Tumor and Immune Cells)
Key Challenge:
Ensuring optimal spatial orientation between the tumor antigen and the immune effector cell (T-cell, NK-cell, macrophage).
Preventing off-target immune activation.
A. Optimized Epitope Positioning for T-Cell Engagers (TCEs)
The distance between CD3 binding (T-cell activation) and tumor antigen binding affects: T-cell activation threshold. Tumor cell recognition efficiency. Cytokine release (risk of CRS - cytokine release syndrome).
Engineering Approaches:
Binding Affinity Tuning Lower CD3 affinity reduces off-target T-cell activation. Higher tumor antigen affinity ensures selectivity. Example: Teclistamab (BCMA/CD3) uses a low-affinity CD3 arm to reduce systemic T-cell activation.
Fc Region Modifications for Half-Life Extension Conventional BiTEs (e.g., Blinatumomab) have no Fc region, resulting in short serum half-life. Solution: Attaching Fc domains or albumin-binding domains. Example: Mosunetuzumab (CD20/CD3) has an IgG-like Fc region for longer stability.
Bispecific Formats That Reduce CRS Heterodimerized Fab arms → Allows selective tumor targeting before T-cell activation. Hinge-region modifications → Control antibody flexibility and epitope spacing.
Example: Optimized Structural Format of a T-Cell Engager
Drug: Blinatumomab (CD19/CD3 BiTE)
Structure: Tandem single-chain variable fragments (scFvs).
Optimization: Low CD3 affinity + no Fc to prevent FcγR-mediated toxicity.
Improvement: Fc-engineered BiTEs (like AMG 562) extend half-life.
Structural Optimization for Enhanced Receptor Clustering and Activation
Key Challenge:
Ensuring correct receptor spacing to enhance signaling without causing paradoxical activation.
A. Tuning Epitope Geometry for Receptor Activation
Distance between Fab arms determines whether receptors are activated or blocked.
Example: CD137 (4-1BB) co-stimulation requires precise clustering to avoid hyperactivation and toxicity.
Engineering Approaches:
Flexible Linkers for Optimal Dimerization Using rigid linkers (e.g., Gly-Ser repeats) to maintain a fixed receptor-binding angle. Example: HER2/HER3 bispecifics (Zenocutuzumab) use an optimal Fab arm angle for enhanced receptor downregulation.
Dual-Receptor Cluster-Inducing Multispecifics Some receptors require clustering at specific membrane microdomains. Example: MCLA-145 (PD-L1/CD137 bispecific) ensures: PD-L1 blockade (immune checkpoint inhibition). CD137 clustering (T-cell activation only in the tumor microenvironment).
Antibody Fragment Fusion for Forced Dimerization Fusing two variable domains into a single Fab for increased receptor crosslinking. Example: RG7716 (Ang2/VEGF CrossMAb) improves anti-angiogenic signaling.
Example: Optimized Structural Format of a Receptor Clustering Antibody
Drug: MCLA-145 (PD-L1/CD137)
Structure: CrossMAb design with domain-swapped Fab arms.
Optimization: Ensures immune checkpoint blockade & T-cell activation occur in the same location.
Improvement: Fine-tuned FcγR binding to avoid systemic T-cell activation.
Structural Optimization for Blocking Multiple Signaling Pathways
Key Challenge:
Preventing tumor escape through compensatory pathways while maintaining selectivity.
Asymmetric Affinity Optimization
Blocking two signaling pathways requires: Balanced affinity (too strong = off-target toxicity; too weak = ineffective). Staggered binding kinetics (slow dissociation for primary target, fast dissociation for secondary).
Engineering Approaches:
Asymmetric Binding Affinity Engineering Example: ABT-122 (TNF-α/IL-17 DVD-Ig). Higher affinity for TNF-α (primary driver of inflammation). Lower affinity for IL-17 (secondary modulation of immune response).
Triple-Targeting to Prevent Adaptive Resistance Some tumors upregulate compensatory pathways when blocked. Example: Tebotelimab (PD-1/LAG-3 bispecific). PD-1 blockade alone leads to LAG-3 overexpression. Dual blockade of PD-1 and LAG-3 prevents T-cell exhaustion.
Fc-Modified Bispecific ADCs (Antibody-Drug Conjugates) Example: Dual-targeted ADCs for HER2/EGFR cancers. One Fab arm binds HER2 (blocking PI3K-AKT signaling). Other Fab arm binds EGFR (blocking MAPK signaling). Fc engineered for controlled drug release.
Example: Optimized Structural Format of a Dual-Signaling Inhibitor
Drug: Tebotelimab (PD-1/LAG-3)
Structure: Fc-engineered IgG-like bispecific.
Optimization: Reduces immune escape by dual-blocking exhaustion markers.
Improvement: Fc modifications to limit systemic immunosuppression.
Structural Optimization for Tumor Microenvironment (TME) Modulation
Key Challenge:
Reprogramming the TME from immunosuppressive (“cold”) to pro-inflammatory (“hot”).
pH-Dependent Antibody Engineering
The tumor microenvironment is acidic (pH ~6.5).
Engineering pH-sensitive Fc domains allows: Antibodies to bind selectively in the TME. Reduced binding in normal tissues, lowering systemic toxicity.
Engineering Approaches:
pH-Selective Tumor-Activated Multispecifics Example: XmAb23104 (PD-1/ICOS bispecific) Binds PD-1 at neutral pH. Binds ICOS only in acidic TME (selective immune activation).
Tumor-Localized FcγR Binding Some macrophages and NK cells are abundant in tumors. Fc modifications allow selective activation in the TME. Example: XmAb20717 (PD-1/CTLA-4 bispecific) Fc engineered for preferential binding to tumor-infiltrating immune cells.
Example: Optimized Structural Format of a TME-Modulating Antibody
Drug: XmAb20717 (PD-1/CTLA-4)
Structure: pH-dependent Fc modification.
Optimization: Activates immune cells only in the tumor.
Improvement: Reduces systemic immune-related toxicities.
By leveraging advanced structural optimizations, multispecific antibodies achieve:
✅ Precise immune cell activation.
✅ Controlled receptor clustering for enhanced signaling.
✅ Dual/multi-pathway inhibition to prevent resistance.
✅ Selective TME activation to reduce off-target toxicity.
Production and Expression Systems
Mammalian expression systems (CHO, HEK293)
Bacterial expression systems (E. coli)
Yeast and insect cell systems
Transgenic animal and plant-based production
Challenges in large-scale manufacturing
Production and Expression Systems for Multispecific Antibodies (msAbs)
The production of multispecific antibodies (msAbs) is significantly more complex than standard monoclonal antibody (mAb) manufacturing due to chain pairing, structural heterogeneity, and stability challenges. Different expression systems are used depending on the complexity, post-translational modifications (PTMs), and scalability requirements.
Mammalian Expression Systems (CHO, HEK293)
Mammalian cell expression is the gold standard for msAb production due to its ability to properly fold proteins, form disulfide bonds, and perform glycosylation.
A. Chinese Hamster Ovary (CHO) Cells
CHO cells are the most widely used system for therapeutic antibody production.
Advantages:
✅ Human-like glycosylation patterns → Essential for proper Fc function (ADCC, CDC).
✅ High protein yield → Efficient antibody secretion.
✅ Scalable for industrial production → Used in commercial antibody manufacturing.
Disadvantages:
❌ Long production times (2-3 weeks for expression).
❌ Expensive culture media (requires FBS-free media).
❌ Risk of high aggregation in complex msAb formats.
Optimizations for msAbs in CHO Cells:
Gene Ratio Optimization Unequal expression of heavy/light chains leads to mispairing. Controlled vector ratios ensure correct pairing (e.g., 2:1 heavy:light ratio for bispecifics).
Fc Engineering to Reduce Aggregation Knob-into-hole technology (to force heterodimerization). CH3 domain modifications for correct pairing.
Stable CHO Cell Lines for Long-Term Production Single-cell cloning techniques. Recombinant gene insertion via CRISPR or PiggyBac transposon systems.
B. Human Embryonic Kidney (HEK293) Cells
HEK293 cells are used for transient expression of multispecific antibodies.
Advantages:
✅ Rapid expression (~1 week) compared to CHO (~2-3 weeks).
✅ Superior protein folding for complex multi-domain msAbs.
✅ Useful for small-scale preclinical production.
Disadvantages:
❌ Lower yield than CHO cells (~10-100 mg/L vs. 1-5 g/L in CHO).
❌ Less scalable for large-scale commercial production.
❌ More heterogeneous glycosylation (higher mannose content).
Optimizations for msAbs in HEK293 Cells:
Use of optimized promoters (e.g., CMV, EF1α) for high expression.
Co-expression of chaperones (PDI, BiP) to improve folding.
Use of exosome-based secretion for improved recovery.
Bacterial Expression Systems (E. coli)
Bacteria, especially E. coli, are used for simple multispecific antibody fragments such as scFv, BiTEs, and nanobody-based msAbs.
Advantages:
✅ Extremely fast production (24-48 hours).
✅ Cost-effective and scalable (high-density fermentation).
✅ Well-characterized system with many expression vectors.
Disadvantages:
❌ No post-translational modifications (e.g., glycosylation, disulfide bonds poorly formed).
❌ Misfolding and inclusion body formation require refolding steps.
❌ Not suitable for full-length IgG-based msAbs.
Optimizations for msAbs in E. coli:
Periplasmic Expression for Correct Folding Directing msAbs to the periplasm (via PelB leader sequence) allows disulfide bond formation.
Cytoplasmic Expression with Redox Control Using engineered Origami™ E. coli strains that allow disulfide bond formation in the cytoplasm.
Refolding from Inclusion Bodies Solubilizing with guanidine HCl, refolding in optimized buffers (e.g., with arginine, glutathione pairs).
Fusion Partner Strategies Trx (thioredoxin), MBP (maltose-binding protein) → Aid folding. His-tag purification for efficient recovery.
Best Use Cases for E. coli Expression:
BiTEs (Blinatumomab)
Single-domain nanobody-based msAbs (Caplacizumab)
Yeast and Insect Cell Systems
Yeast Expression Systems (Pichia pastoris, Saccharomyces cerevisiae)
Yeast is used for msAbs that require proper glycosylation but not human-like complexity.
Advantages:
✅ Faster than CHO (~5-7 days instead of 2-3 weeks).
✅ Higher yield than HEK293 (~1-2 g/L).
✅ Secretes correctly folded proteins into the medium.
Disadvantages:
❌ Hypermannosylation alters antibody function.
❌ Limited ability to express full-length IgG.
Optimizations for msAbs in Yeast:
Engineering human-like glycosylation pathways (e.g., GlycoSwitch® system).
Using Pichia pastoris for higher secretion efficiency.
Insect Cell Expression (Sf9, Sf21, Hi5)
Insect cells (via baculovirus expression systems) are used for complex multi-domain proteins.
Advantages:
✅ Post-translational modifications similar to mammalian cells.
✅ High yield in large-scale suspension cultures.
✅ Lower cost than CHO cells.
Disadvantages:
❌ Non-human glycosylation (high-mannose, α(1,3)-fucose residues).
❌ Long production cycle (2-3 weeks).
Optimizations for msAbs in Insect Cells:
Using Lec13 insect cell lines to improve glycosylation.
Engineering baculovirus promoters for high-level expression.
Best Use Cases:
Bispecific nanobody-fusion proteins
Complex msAb fragments with multiple domains
Transgenic Animal and Plant-Based Production
Transgenic Animal Expression (Goats, Cows, Chickens)
Antibodies secreted into milk or eggs.
Example: Atryn (antithrombin) from goat milk.
Advantages:
✅ Very high yield (~10 g/L in milk).
✅ Cost-effective once established.
Disadvantages:
❌ Difficult to control glycosylation.
❌ High initial cost of transgenic animal generation.
Plant-Based Expression (Tobacco, Rice, Maize)
Antibodies produced in plant cells using Agrobacterium-mediated transformation.
Example: Zmapp (Ebola antibody) from tobacco.
Advantages:
✅ Scalable and cost-effective.
✅ No risk of mammalian pathogen contamination.
Disadvantages:
❌ Plant glycans differ from human glycans.
❌ Longer development timeline than CHO or HEK.
Challenges in Large-Scale Manufacturing
Misfolding and Aggregation → Requires extensive solubility optimization.
Light and Heavy Chain Mispairing → Solved with Knob-into-Hole or CrossMAb technology.
Glycosylation Consistency → Requires humanized glycoengineering.
Scalability of Novel msAb Formats → Bioreactor conditions must be optimized per format.
Mechanisms of Action in Disease Treatment
Cancer Immunotherapy T-cell redirection (CD3-engaging bispecifics) Checkpoint inhibition (PD-1/CTLA-4 combination) Tumor-associated antigen (TAA) targeting Antibody-drug conjugates (ADCs) with multispecificity
Autoimmune and Inflammatory Diseases Dual cytokine targeting (e.g., IL-17/IL-23) Modulating Tregs and effector T-cell balance
Infectious Diseases Neutralization of multiple viral epitopes (e.g., HIV, COVID-19) Enhanced pathogen clearance mechanisms
Neurodegenerative Diseases Multi-targeted amyloid-beta and tau antibodies
Crossing the blood-brain barrier (BBB)
Mechanisms of Action of Multispecific Antibodies (msAbs) in Disease Treatment
Multispecific antibodies (msAbs) have transformed cancer therapy, autoimmune disease treatment, infectious disease management, and neurodegenerative disease intervention by engaging multiple targets with a single molecule. Below is a deep technical breakdown of their mechanisms of action in various disease categories.
Cancer Immunotherapy
Cancer immunotherapy harnesses the immune system to identify and eliminate tumor cells. Multispecific antibodies enhance this process through T-cell redirection, checkpoint inhibition, tumor antigen targeting, and antibody-drug conjugates (ADCs).
T-Cell Redirection (CD3-Engaging Bispecifics)
Mechanism of Action
Bispecific T-cell engagers (BiTEs) simultaneously bind: CD3ε on T cells → Activates T-cell receptor (TCR) signaling. Tumor-associated antigens (TAAs) on cancer cells → Directs cytotoxic T-cell attack.
Without requiring MHC presentation, BiTEs force T cells to kill tumor cells by:
1. Inducing immune synapse formation between T cells and tumors.
2. Triggering TCR signaling, leading to the release of granzyme B and perforin.
3. Promoting tumor cell apoptosis via Fas-FasL interactions.
Key Engineering Strategies
Affinity Tuning → Reducing CD3 affinity to avoid non-specific T-cell activation.
Half-Life Extension → Fc engineering to increase stability in plasma.
Tumor-Associated Antigen (TAA) Targeting
Many tumors overexpress specific antigens that can be targeted using bispecific antibodies.
Mechanism of Action
Multispecific antibodies bind two tumor-specific epitopes, increasing specificity.
By crosslinking receptors, they can enhance tumor suppression.
They recruit immune effector cells (NK cells, macrophages) to mediate antibody-dependent cell cytotoxicity (ADCC).
Antibody-Drug Conjugates (ADCs) with Multispecificity
Multispecific
Tumor antigen targeting ensures ADC internalization.
Endosomal processing releases cytotoxic drugs inside tumor cells.
By targeting two TAAs, specificity is enhanced, reducing off-target effects.
Autoimmune and Inflammatory Diseases
Multispecific antibodies provide
IL-17 and IL-23 drive autoimmune inflammation.
Dual inhibitors simultaneously neutralize both cytokines, preventing disease progression.
Modulating Tregs and Effector T-Cell Balance
Some autoimmune diseases result from Treg depletion and excessive effector T-cell activation.
Multispecific antibodies can restore Treg balance by: Blocking pro-inflammatory pathways (PD-1, LAG-3). Promoting IL-2-dependent Treg expansion.
Example
Tebotelimab (PD-1/LAG-3 bispecific): Restores immune balance.
Infectious Diseases
Multispecific antibodies enhance pathogen neutralization and clearance.
Neutralization of Multiple Viral Epitopes
Viruses mutate rapidly, evading monoclonal antibodies.
Multispecific antibodies target conserved viral regions, preventing escape.
Enhanced Pathogen Clearance Mechanisms
Recruiting immune cells for Fc-mediated opsonization.
Engaging complement system (CDC).
Blocking viral entry via ACE2-mimetic antibodies.
Neurodegenerative Diseases
Multi-Targeted Amyloid-Beta and Tau Antibodies
Amyloid-beta (Aβ) plaques and Tau tangles drive Alzheimer’s disease.
Bispecific antibodies can: Bind multiple Aβ isoforms → Prevent plaque aggregation. Simultaneously target Tau → Halt neurofibrillary tangle formation.
Crossing the Blood-Brain Barrier (BBB)
The BBB prevents antibody penetration into the CNS.
Multispecific BBB-crossing antibodies: Use transferrin receptor (TfR) targeting to shuttle across. Have Fc-modifications to increase CNS permeability.
Multispecific antibodies revolutionize treatment strategies by enabling:
✅ Precise immune modulation.
✅ Simultaneous blockade of multiple disease pathways.
✅ Efficient CNS delivery for neurodegeneration.
Challenges and Limitations
Manufacturing complexity and cost
Immunogenicity and off-target effects
Stability and aggregation issues
Bispecific vs. combination therapy considerations
Intellectual property and patent landscape
Challenges and Limitations of Multispecific Antibodies (msAbs)
Despite their immense therapeutic potential, multispecific antibodies (msAbs) face significant technical, biological, and regulatory challenges. Below is a deep technical breakdown of the key limitations in msAb development, along with possible engineering solutions.
Manufacturing Complexity and Cost
One of the biggest hurdles in multispecific antibody production is the increased complexity in molecular design and large-scale manufacturing. Unlike monoclonal antibodies (mAbs), msAbs require:
Precise heavy and light chain pairing.
Higher-order structural stability.
Post-translational modifications (PTMs) that can affect function.
Key Manufacturing Challenges
Engineering Solutions
Heavy-Light Chain Pairing Optimization Knob-into-Hole Technology (KiH) – Ensures correct heterodimerization. CrossMAb Engineering – Prevents incorrect light chain pairing. Asymmetric Fc Engineering – Promotes heterodimer formation.
Cell Line Engineering for Higher Expression CHO cell gene amplification strategies (e.g., DHFR and GS systems). Co-expression vector tuning → Ensures correct stoichiometry.
Glycoengineering for Batch Consistency Use of GlycoSwitch® technology for homogeneous glycosylation. CRISPR/Cas9-based genome editing to modify glycosylation pathways.
Cost Implications
Manufacturing costs are 2-5x higher than traditional mAbs.
Yield optimization and scalable purification strategies are critical for commercial viability.
Immunogenicity and Off-Target Effects
Immunogenicity is a major concern for multispecific antibodies, as novel scaffold designs introduce non-natural epitopes that can trigger anti-drug antibody (ADA) responses.
Mechanisms of Immunogenicity
Non-Human Sequences in Engineered Regions Novel fusion domains or modified Fabs can introduce foreign epitopes. Murine-derived single-domain antibodies (nanobodies) have higher immunogenic potential.
Off-Target Binding Due to Multispecificity Binding to low-affinity unintended targets leads to toxicity. Example: CD3-bispecific antibodies can activate T cells non-specifically, leading to cytokine release syndrome (CRS).
FcγR-Mediated Unwanted Activation Some bispecific formats can bind Fcγ receptors (FcγRs) on immune cells, leading to off-target immune activation.
Engineering Solutions
Humanization of Non-Human Domains AI-based epitope mapping to predict immunogenic regions. CDR grafting and human framework selection.
Reducing Off-Target Effects Affinity tuning → Engineering variable domains to favor primary targets over secondary off-targets. Tumor microenvironment activation → Engineering pH-dependent conditional activation antibodies.
Fc Engineering to Minimize Unwanted Activation Fc-silencing mutations (L234A, L235A, P329G) reduce FcγR binding. Bispecific antibodies with modified hinge regions to reduce unwanted crosslinking.
Example of Fc Optimization
XmAb20717 (PD-1/CTLA-4 bispecific) uses Fc mutations to reduce toxicity while maintaining checkpoint blockade efficiency.
Stability and Aggregation Issues
Multispecific antibodies
Misfolding.
Aggregation (high molecular weight species).
Shorter half-life due to instability.
Key Factors Affecting Stability
Engineering Solutions
Reducing Aggregation Hydrophilic mutation scanning to reduce hydrophobic patches. Charge engineering to optimize isoelectric point (pI).
Enhancing Structural Stability Disulfide bond engineering for domain stabilization. AI-based molecular dynamics simulations to predict aggregation-prone regions.
Half-Life Extension Strategies Fc modifications for neonatal Fc receptor (FcRn) binding enhancement. PEGylation or albumin fusion to increase serum stability.
Example of Stability Optimization
Tebotelimab (PD-1/LAG-3 bispecific) uses structure-guided engineering to improve solubility and shelf-life.
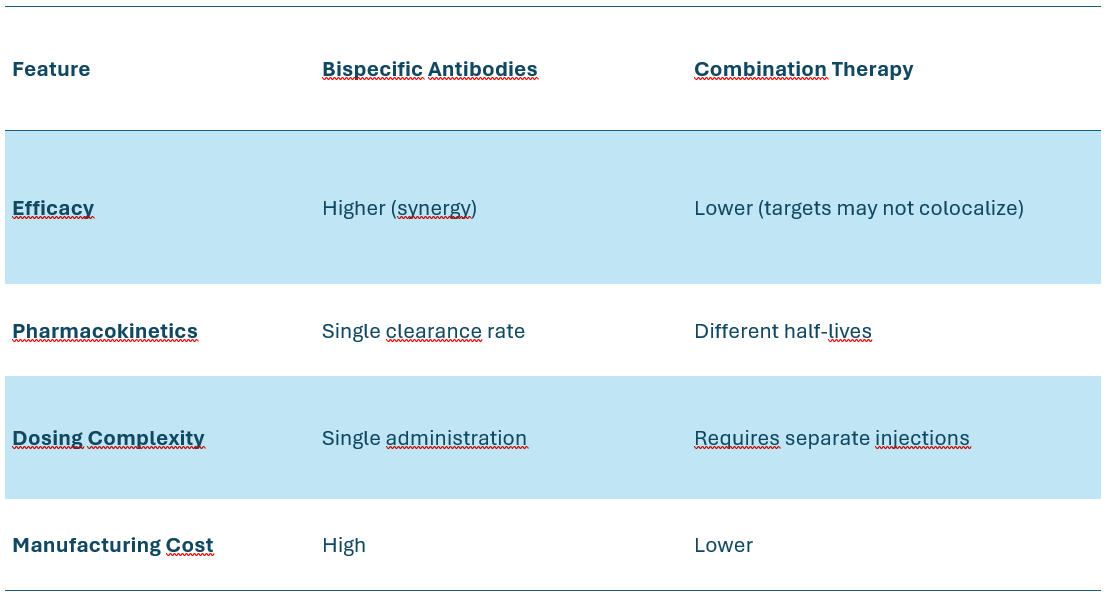
Bispecific vs. Combination Therapy Considerations
A major challenge in msAb therapy is determining whether a bispecific design is superior to simply combining two monoclonal antibodies.
Comparing Bispecifics vs. Combination Therapy
Key Considerations
If targets must be co-engaged, a bispecific design is preferable (e.g., T-cell engagers).
If targets work independently, combination therapy is more flexible.
Example: PD-1/CTLA-4
Ipilimumab (CTLA-4) + Nivolumab (PD-1) combination therapy works, but bispecific XmAb20717 achieves the same effect with lower toxicity.
Multispecific antibodies offer revolutionary therapeutic potential, but face substantial challenges in:
✅ Manufacturing complexity → Requires advanced protein engineering.
✅ Immunogenicity risks → Must undergo epitope screening.
✅ Stability issues → Needs aggregation-resistant designs.
✅ Regulatory and IP hurdles → Companies must navigate patent constraints.
Next-Generation Multispecific Antibody Technologies
AI-driven antibody design
Synthetic biology and directed evolution
Nanobody-based multispecific platforms
Engineered Fc domains for extended half-life
CRISPR and gene-editing approaches for antibody optimization
Next-Generation Multispecific Antibody (msAb) Technologies
The evolution of multispecific antibodies (msAbs) has accelerated due to advancements in AI-driven design, synthetic biology, directed evolution, nanobody-based platforms, Fc engineering, and CRISPR-mediated optimization. These cutting-edge technologies address key challenges in stability, specificity, manufacturability, and therapeutic efficacy.
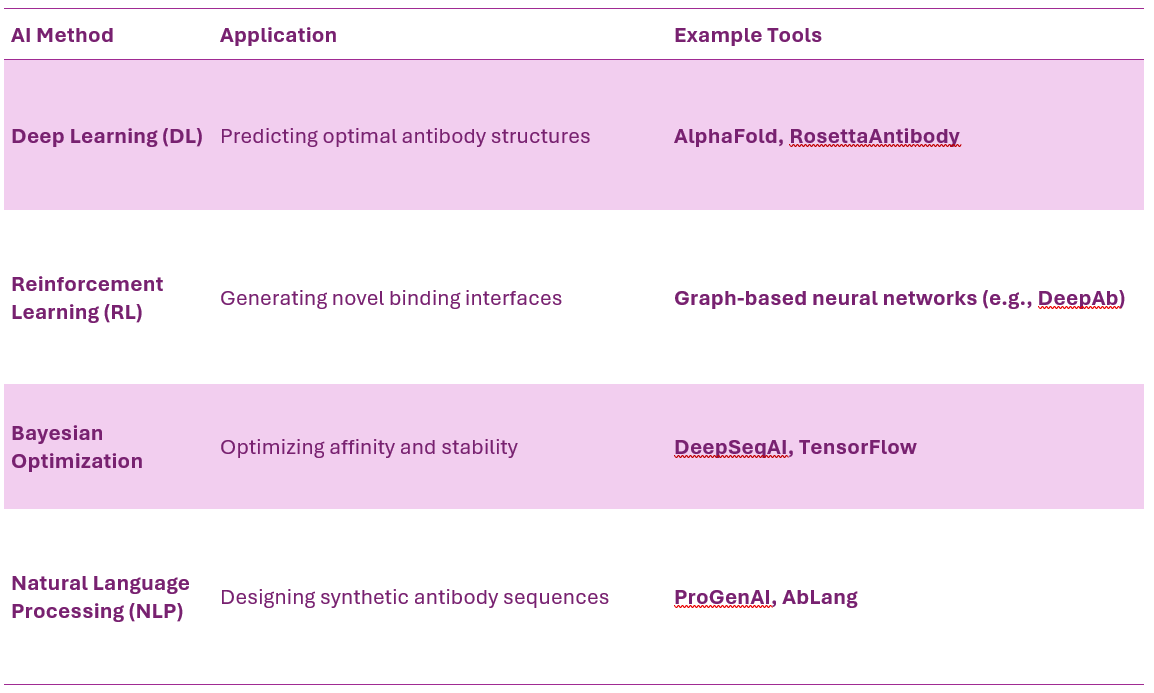
AI-Driven Antibody Design
Why Use AI in Antibody Engineering?
Traditional antibody discovery relies on animal immunization, phage display, and rational design, which are time-consuming.
AI-based methods leverage machine learning (ML), deep learning (DL), and structural modeling to predict, optimize, and generate high-affinity and stable multispecific antibodies.
AI-Driven Approaches in msAb Design
Key AI-Enabled Engineering Strategies
De Novo Antibody Design AI-generated antibody sequences with high binding affinity, solubility, and manufacturability. Example: Generate high-affinity CD3-binding domains for T-cell engagers with reduced off-target effects.
AI-Based Epitope and Paratope Prediction ML algorithms scan millions of antibody-antigen interactions to identify optimal binding sites. Example: Absci’s AI-driven discovery of next-gen TAA/CD3 bispecifics.
AI-Guided Affinity Maturation Reinforcement learning optimizes CDR loops to enhance binding strength while maintaining stability. Example: AI-optimized HER2/HER3 bispecific CrossMAb with enhanced tumor selectivity.
Synthetic Biology and Directed Evolution
Synthetic Biology for Multispecific Antibodies
Synthetic biology allows for the rational engineering of synthetic antibody scaffolds beyond traditional IgG structures.
Custom-built antibody scaffolds can be designed with: Non-natural amino acids for enhanced stability. Engineered binding pockets for multi-epitope targeting. Self-assembling multispecific domains.
Directed Evolution in Antibody Engineering
Directed evolution mimics natural selection by creating massive antibody libraries and iteratively selecting high-affinity binders.
Key Directed Evolution Strategies
Breakthroughs Using Synthetic Biology
Thermostable T-cell engagers (TCEs) → Engineered via directed evolution to resist aggregation at physiological temperatures.
Self-assembling bispecific nanobodies → Designed using computational synthetic biology.
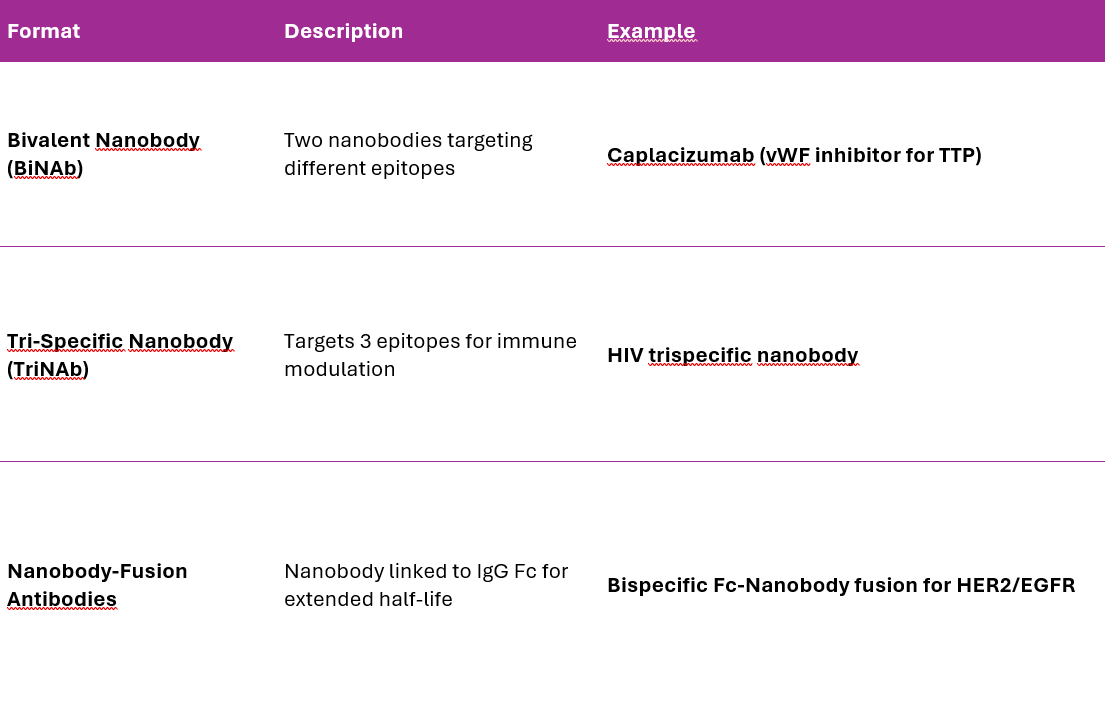
Nanobody-Based Multispecific Platforms
Why Use Nanobody-Based msAbs?
Nanobodies (VHHs) are derived from camelid heavy-chain antibodies and are 10x smaller than conventional IgGs.
Nanobody-based msAbs offer: Higher tissue penetration (ideal for brain tumors, solid tumors). Greater modularity for multispecific design. Lower immunogenicity and improved manufacturability.
Types of Nanobody-Based Multispecifics
Recent Advances in Nanobody Engineering
Hinge region optimization → Improves flexibility and target engagement.
High-affinity nanobody libraries → Generated using synthetic yeast display.
Dual-variable domain nanobody formats → Enhanced for multi-pathogen neutralization (e.g., HIV, SARS-CoV-2, Influenza).
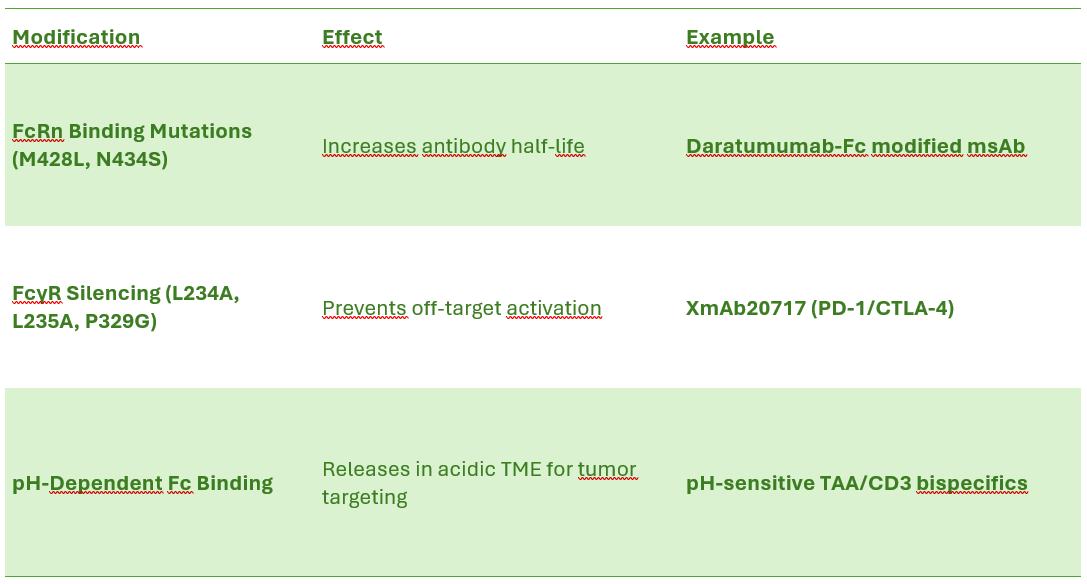
Engineered Fc Domains for Extended Half-Life
Why Engineer the Fc Region?
The Fc region determines antibody half-life, effector function, and clearance.
Native IgG Fc binds FcRn, allowing antibody recycling and extended serum half-life.
Multispecific antibodies often require Fc engineering to: Avoid unwanted FcγR binding (prevents off-target immune activation). Enhance FcRn binding (extends circulation time). Enable tumor-selective immune engagement.
Fc Engineering Strategies
Next-Gen Applications
Tumor-activated Fc switches → Conditional Fc engagement only in tumor microenvironments.
Self-assembling Fc-fusion msAbs → Extended half-life without aggregation.
CRISPR and Gene-Editing Approaches for Antibody Optimization
How CRISPR is Revolutionizing Antibody Discovery
Traditional antibody development is slow and requires animal models.
CRISPR/Cas9 enables precision engineering of CHO cells, hybridomas, and synthetic antibody libraries.
CRISPR Applications in msAb Development
Gene Knock-Ins for Enhanced Antibody Expression CRISPR-based CHO cell optimization to enhance antibody secretion. Insertion of human-like glycosylation pathways to reduce immunogenicity.
Optimizing Heavy-Light Chain Pairing CRISPR-edited CHO cell lines with enforced light chain co-expression. Pre-programmed gene knockouts to remove undesired mispairing variants.
CRISPR-Guided Affinity Maturation High-throughput CRISPR screens to identify antibody variants with optimal binding. Directed mutagenesis of CDR regions using base-editing techniques.
Recent Breakthroughs
CRISPR-based synthetic phage display → Rapid evolution of high-affinity bispecific antibodies.
CRISPR-modified glycoengineered CHO cells → Ensure batch consistency in antibody glycosylation.
Next-generation multispecific antibodies leverage AI, synthetic biology, nanobody platforms, Fc engineering, and CRISPR technologies to:
✅ Enhance therapeutic efficacy and stability.
✅ Improve manufacturability and half-life.
✅ Enable precision immune modulation.
Conclusion: The Future of Multispecific Antibodies
Multispecific antibodies (msAbs) have emerged as a transformative advancement in antibody engineering, offering unparalleled precision and efficacy in therapeutic applications. By engaging multiple targets simultaneously, msAbs overcome the limitations of traditional monoclonal antibodies, providing enhanced immune modulation, improved tumor targeting, and reduced resistance mechanisms. The ability to simultaneously block multiple disease pathways, recruit immune effector cells, or enhance receptor clustering makes msAbs a powerful tool in cancer immunotherapy, autoimmune disease treatment, infectious disease management, and neurodegenerative research.
Despite their immense potential, the development and manufacturing of msAbs remain complex, requiring sophisticated engineering strategies to ensure correct heavy-light chain pairing, stability, and manufacturability. Advances such as Knob-into-Hole technology, CrossMAb scaffolds, and AI-driven antibody discovery have significantly improved msAb design, while CRISPR-based cell line optimization and glycoengineering are enhancing production efficiency and therapeutic safety. However, challenges such as immunogenicity, aggregation, and off-target effects must still be carefully addressed to fully harness the clinical potential of msAbs.
As the field progresses, the integration of synthetic biology, AI-driven computational modeling, and next-generation expression systems will further accelerate msAb innovation. Emerging formats such as trispecific and tetraspecific antibodies, antibody-drug conjugates (ADCs), and nanobody-based multispecifics are expanding the therapeutic landscape, allowing for more precise and personalized treatments. The convergence of these cutting-edge technologies will not only refine existing therapies but also unlock new possibilities for tackling complex and refractory diseases.
Looking ahead, multispecific antibodies are poised to become a cornerstone of modern biopharmaceutical development. With continued advancements in molecular engineering, computational optimization, and large-scale manufacturing, msAbs will redefine the future of targeted therapies. As more next-generation msAbs enter clinical trials and receive regulatory approval, they will provide new hope for patients facing cancers, autoimmune disorders, infectious diseases, and neurodegenerative conditions. The journey of msAbs is just beginning, and their potential to revolutionize medicine is vast and promising.
Key Takeaways from This Article:
Multispecific Antibodies (msAbs) Are a Major Advancement in Immunotherapy
Technological Innovations Have Overcome Many Early Challenges
Expanding Applications in Cancer, Autoimmune, and Infectious Diseases
Challenges in Manufacturing and Stability Are Being Addressed
The Future of msAbs is Bright with Next-Generation Developments
The field of multispecific antibody engineering is evolving rapidly, and its potential impact on medicine is profound. Continued research and innovation will be key to unlocking the full potential of these powerful biologics, ensuring they become a mainstay in modern therapeutic strategies.