Understanding RNA-Based Therapies: From Antisense Oligonucleotides to mRNA
Nucleic Acid Therapeutics, antisense oligonucleotides, siRNA, miRNA, RNA aptamers, Ribozymes, mRNA Therapeutics
Nucleic acid-based therapeutics represent a groundbreaking frontier in modern medicine, utilizing the precise targeting capabilities of synthetic or naturally occurring DNA and RNA molecules to modulate gene expression. These therapies, also referred to as oligonucleotide therapeutics or gene-silencing technologies, interact directly with specific RNA or DNA sequences to influence cellular function, offering unprecedented opportunities to treat a wide range of diseases. By either silencing harmful genes, enhancing beneficial ones, or modifying how genetic messages are processed, these molecules provide innovative solutions for genetic disorders, cancers, infectious diseases, and neurodegenerative conditions. As a cornerstone of personalized medicine, nucleic acid-based therapies hold the potential to address the root causes of diseases that were previously considered untreatable, shifting the focus from symptom management to precise genetic intervention.
This emerging field encompasses a variety of therapeutic modalities, each with distinct mechanisms of action. Antisense oligonucleotides (ASOs) are designed to bind complementary RNA sequences, effectively blocking or altering protein production, while small interfering RNAs (siRNAs) utilize the RNA interference (RNAi) pathway to degrade specific mRNA targets with high precision. Other strategies, such as microRNAs (miRNAs), exploit naturally occurring gene-regulating molecules to fine-tune cellular processes. RNA aptamers, short RNA or DNA sequences with antibody-like binding properties, target proteins with exceptional specificity, while ribozymes function as catalytic RNA molecules to cleave specific RNA sequences. Meanwhile, mRNA therapeutics, exemplified by the widely used COVID-19 vaccines, enable cells to produce therapeutic proteins directly, opening new avenues in vaccines, cancer immunotherapy, and enzyme replacement therapy. Collectively, these approaches exemplify the power of nucleic acids as versatile tools in molecular medicine.
While the potential of nucleic acid-based therapeutics is immense, their practical application comes with challenges. Delivery systems, such as lipid nanoparticles, are critical for protecting these molecules and ensuring their uptake by target cells, but achieving efficient and tissue-specific delivery remains an ongoing hurdle. Stability is another concern, as unmodified nucleic acids are prone to rapid degradation by enzymes in the body. Advances in chemical modifications, including the incorporation of modified nucleotides and protective structures, have significantly improved their stability and functionality. Additionally, minimizing off-target effects to ensure precision and reduce unintended interactions is a key area of research. Despite these obstacles, the field continues to advance rapidly, driven by innovations in molecular biology, chemistry, and nanotechnology. With these strides, nucleic acid-based therapeutics are poised to transform medicine by addressing the molecular underpinnings of disease and offering highly targeted, effective, and customizable treatments for a wide array of conditions.
Subcategories Within Nucleic Acid-Based Therapeutics
Synthetic or naturally occurring nucleic acids (DNA or RNA) can be used therapeutically to modulate gene expression. These molecules work by interacting with RNA or DNA sequences to achieve a therapeutic effect. Also known as oligonucleotide therapeutics" or "gene-silencing technologies." This term highlights their shared goal: to modulate gene expression, either by turning genes off, enhancing their activity, or modifying how their messages are processed.
Antisense Oligonucleotides (ASOs): Synthetic, single-stranded DNA or RNA molecules that bind to complementary RNA sequences to block or modify gene expression.
Small Interfering RNAs (siRNAs): Double-stranded RNA molecules that use the RNA interference (RNAi) pathway to silence specific mRNA targets.
MicroRNAs (miRNAs): Naturally occurring small RNAs that regulate gene expression by binding to complementary mRNA sequences and preventing their translation or inducing degradation.
RNA Aptamers: Short RNA or DNA sequences that fold into specific shapes to bind and inhibit target proteins, similar to antibodies.
Ribozymes: Catalytic RNA molecules designed to cleave specific RNA sequences.
mRNA Therapeutics: Synthetic mRNAs used to produce therapeutic proteins (e.g., mRNA vaccines like those for COVID-19).
Antisense Oligonucleotides (ASOs)
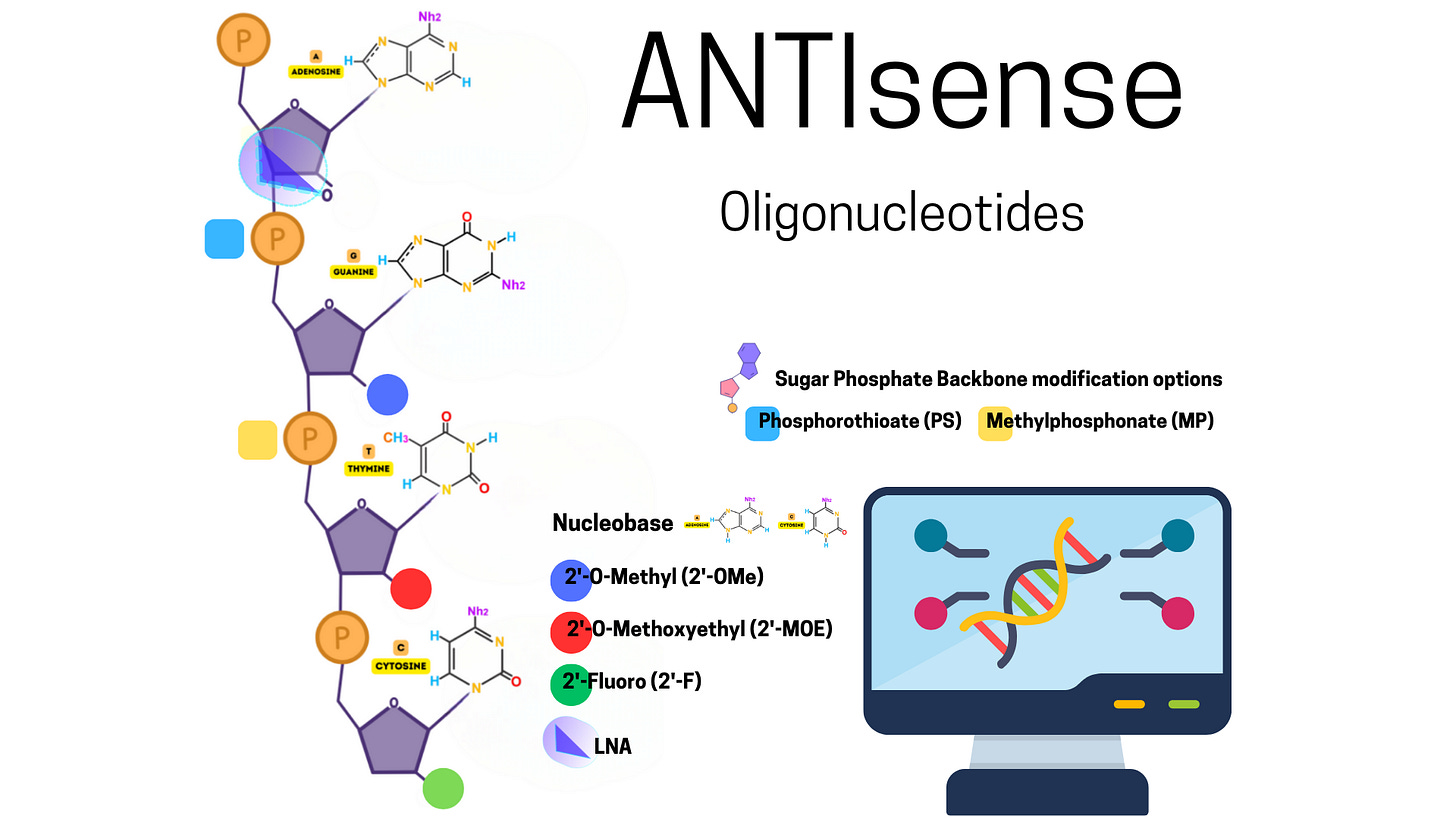
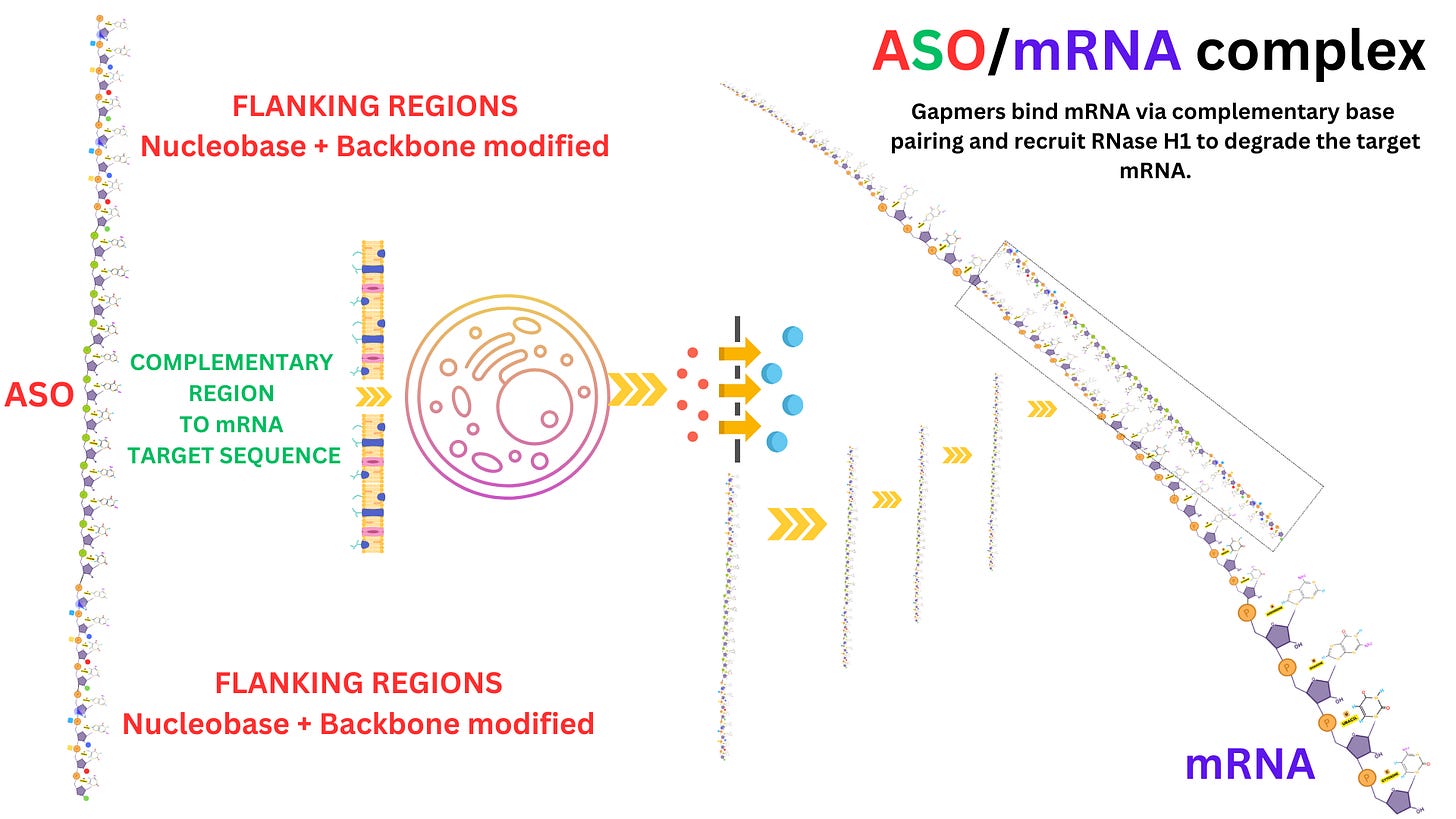
Antisense oligonucleotides (ASOs) are short, single-stranded DNA or RNA molecules designed to bind to specific RNA sequences through complementary base pairing. They are synthetic molecules engineered to interfere with RNA's normal function, usually by modulating gene expression.
How Are ASOs Structured?
ASOs are chemically modified to enhance their stability and effectiveness in biological environments. Without modifications, unmodified oligonucleotides are rapidly degraded by nucleases (enzymes that break down nucleic acids).
Key structural features include:
Length: Typically 12–25 nucleotides long, optimized for specificity and functionality.
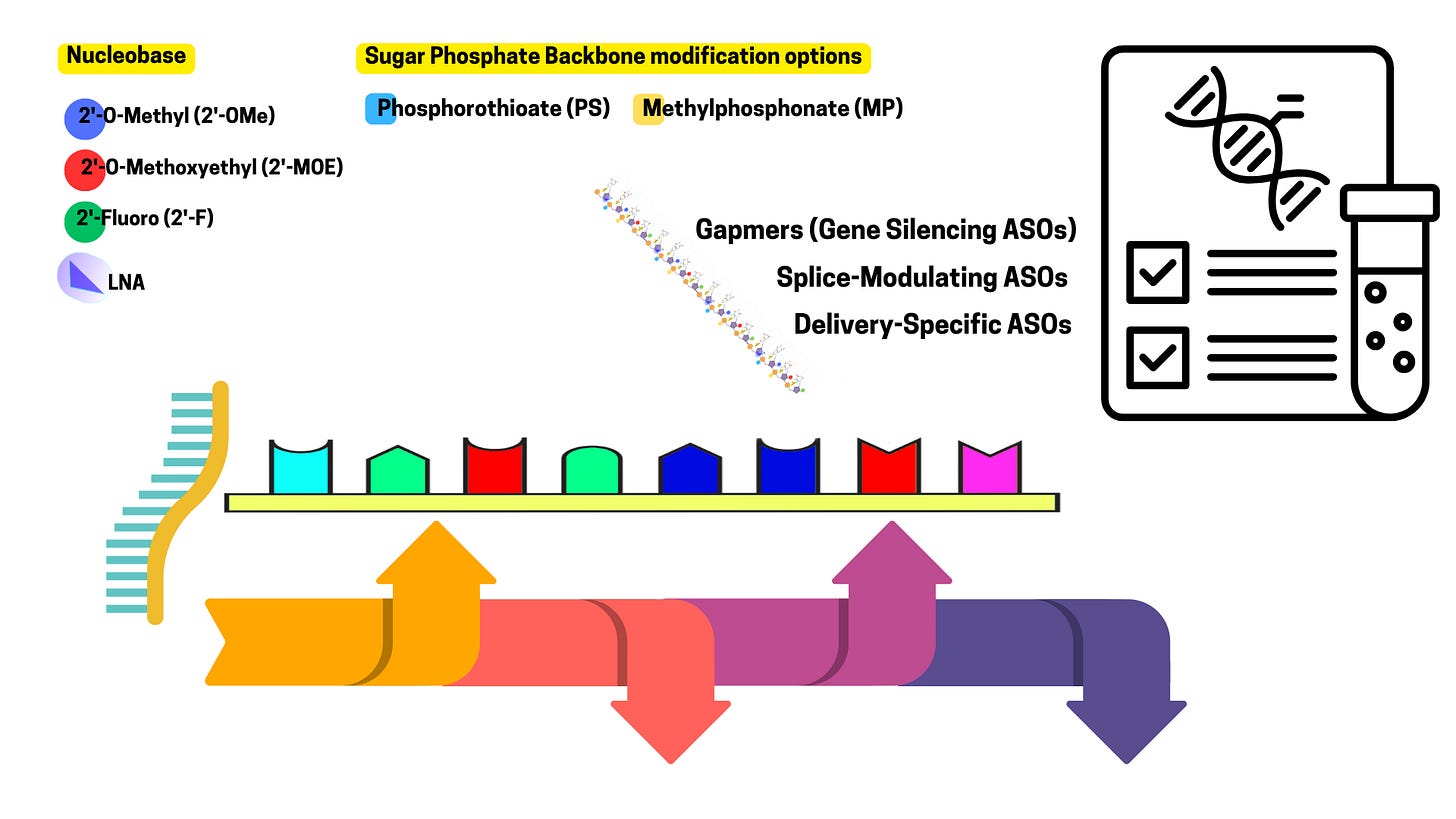
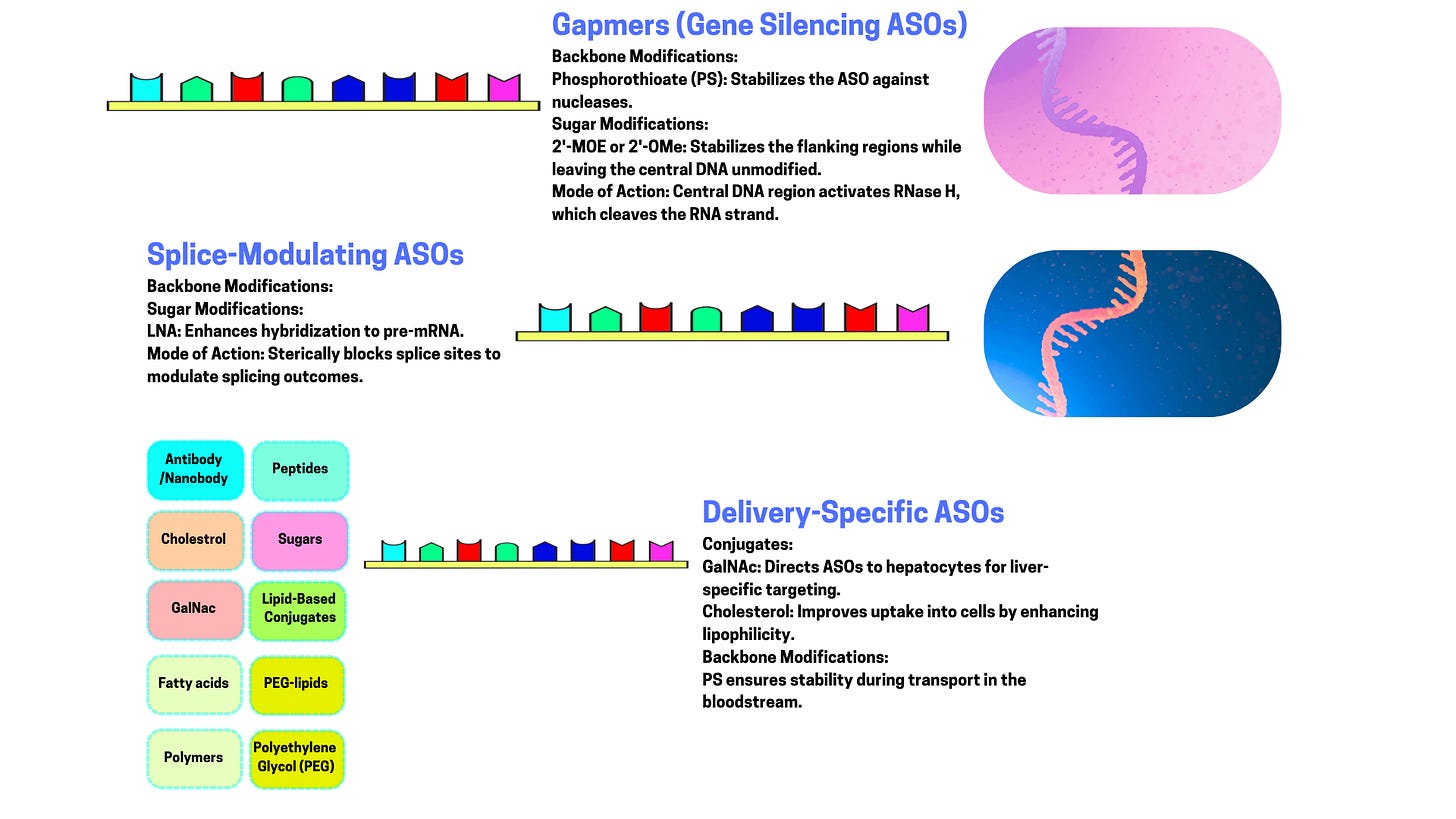
Modifications: To improve resistance to degradation, reduce immune activation, and increase binding affinity: Phosphorothioate Backbone: One oxygen in the phosphate group is replaced with sulfur, making the ASO more nuclease-resistant. 2’-Modifications: Alterations on the sugar ring (e.g., 2’-O-methyl or 2’-O-methoxyethyl) to enhance stability and reduce off-target effects. Locked Nucleic Acids (LNAs): Constrained sugar conformations for higher binding affinity.
Conjugation: Some ASOs are conjugated to delivery molecules (e.g., lipids or peptides) to improve cellular uptake.
How Do ASOs Work?
ASOs interact with their target RNA in specific ways, depending on their design. Their mechanism of action can be grouped into three main categories:
1. RNA Cleavage via RNase H
Mechanism: The ASO binds to a complementary mRNA sequence, forming a DNA-RNA hybrid. This hybrid is recognized by RNase H, an enzyme that cleaves the RNA strand, leaving the DNA strand intact.
Effect: The targeted mRNA is destroyed, preventing its translation into protein.
2. Steric Hindrance (Translation Blockade)
Mechanism: The ASO binds to a specific region of mRNA, such as the start codon or ribosome binding site, physically blocking the machinery (ribosome) from translating the mRNA.
Effect: The mRNA remains intact but cannot produce the corresponding protein.
3. Splicing Modulation
Mechanism: ASOs can bind to pre-mRNA (the initial transcript before splicing) and mask splice sites or regulatory sequences, altering the splicing pattern.
Effect: The resulting mRNA may exclude certain exons, include skipped ones, or shift the reading frame, producing a modified or truncated protein.
Applications of ASOs
ASOs are versatile tools for research and therapeutics, especially in targeting diseases caused by abnormal or overactive genes.
1. Therapeutic Applications
Genetic Disorders: ASOs are used to correct or compensate for genetic defects: Example: Nusinersen (Spinraza) for spinal muscular atrophy modifies splicing of the SMN2 gene to produce functional protein.
Cancer: Target oncogenes or genes involved in tumor growth. Example: Inhibiting BCL2, an anti-apoptotic protein, in cancer cells.
Infectious Diseases: Target viral RNA to prevent replication.
Neurodegenerative Disorders: Silence genes contributing to toxic protein accumulation. Example: ASOs targeting HTT mRNA in Huntington’s disease to reduce mutant huntingtin protein.
2. Research Tools
ASOs are extensively used in molecular biology to:
Silence genes in vitro (gene knockdown studies).
Probe RNA function and splicing mechanisms.
Advantages of ASOs
High Specificity: Designed to bind only to the intended RNA sequence.
Customizability: Can target virtually any RNA sequence, including "undruggable" targets.
Broad Applications: Useful in treating diseases caused by gene overexpression, splicing errors, or toxic RNA production.
Challenges and Limitations
Delivery: Getting ASOs to their target tissue or cells is a significant challenge. Solutions: Lipid nanoparticles, conjugation to cell-penetrating peptides, or GalNAc ligands for liver targeting.
Off-Target Effects: Imperfect binding can lead to unintended interactions.
Stability and Immunogenicity: Despite modifications, ASOs may still trigger immune responses or degrade too quickly.
Cost: Synthesis and large-scale production remain expensive.
Future Directions
Enhanced delivery systems: e.g., nanoparticles or exosome-based carriers.
Novel backbone and sugar modifications to improve stability and reduce side effects.
Expanded use in personalized medicine: Designing ASOs tailored to individual genetic profiles.
Small interfering RNAs (siRNAs)
siRNAs are integral to the RNA interference (RNAi) pathway, a highly conserved cellular mechanism used to regulate gene expression by targeting and degrading specific mRNA molecules.
1. What Are siRNAs?
siRNAs are short, double-stranded RNA (dsRNA) molecules, typically 21-23 nucleotides in length. They are either:
Synthetic: Designed and synthesized in the lab for therapeutic or experimental purposes.
Endogenous: Derived from long dsRNAs or RNA precursors that are processed in the cell.
The primary function of siRNAs is to silence gene expression by directing the degradation of complementary mRNA sequences in a highly sequence-specific manner.
2. siRNA Structure
siRNAs are composed of:
Two RNA strands: Guide strand (antisense strand): Complementary to the target mRNA; this strand directs the RNA-induced silencing complex (RISC) to the mRNA. Passenger strand (sense strand): Complementary to the guide strand; it is usually degraded during RISC loading.
3’ Overhangs: Two unpaired nucleotides on the 3’ ends of both strands, enhancing binding efficiency to RISC.
Length: 19-21 base-paired nucleotides with the 3’ overhangs.
Example of a canonical siRNA:
Guide strand: 5' - ACUGGCUAAUCCGAUGCUA - 3'
Passenger: 3' - UGAUCCGAUUAGGCUACGAU - 5'
3. How Do siRNAs Work?
The siRNA mechanism of action involves several steps, taking place within the RNA interference (RNAi) pathway. Here’s how it works:
Step 1: siRNA Delivery and Entry
siRNAs are delivered into the cytoplasm through various methods, such as lipid nanoparticles or chemical modifications that promote stability and cellular uptake.
Step 2: Loading into RISC
In the cytoplasm, the siRNA duplex is recognized and loaded into the RNA-induced silencing complex (RISC).
Argonaute 2 (AGO2), the catalytic protein within RISC, plays a key role. It: Selects the guide strand of the siRNA based on strand thermodynamic stability. Discards the passenger strand by degradation or displacement.
Step 3: Target mRNA Binding
The RISC-siRNA complex scans cytoplasmic mRNAs for sequences complementary to the guide strand.
Target recognition occurs through Watson-Crick base pairing between the siRNA guide strand and the mRNA.
Step 4: mRNA Cleavage
Once the siRNA guide strand binds the mRNA, AGO2 acts as an endonuclease, cleaving the mRNA at the complementary site.
Cleaved mRNA is further degraded by cellular exonucleases, preventing translation into protein.
Step 5: Recycling of RISC
After mRNA cleavage, the RISC complex is recycled and can engage additional mRNA molecules, amplifying the silencing effect.
4. Applications of siRNAs
siRNAs are highly versatile tools used in both research and therapeutics. Their ability to silence specific genes with high precision makes them invaluable in many contexts.
A. Research Applications
Gene Knockdown Studies: siRNAs are widely used in laboratories to temporarily reduce the expression of target genes, enabling functional studies. Example: Identifying the role of a gene in cancer progression by silencing it in cell lines.
Pathway Analysis: siRNAs help dissect complex cellular pathways by selectively silencing specific genes.
B. Therapeutic Applications
siRNAs are increasingly being developed as drugs to target diseases at the mRNA level. Some examples include:
Metabolic Disorders: Patisiran (Onpattro): The first FDA-approved siRNA drug, used to treat hereditary transthyretin-mediated amyloidosis by silencing the gene encoding transthyretin (TTR).
Cancer: siRNAs can target oncogenes, tumor-suppressor inhibitors, or genes involved in drug resistance.
Viral Infections: siRNAs can inhibit viral replication by targeting viral RNA or host factors essential for the viral life cycle.
Neurodegenerative Diseases: siRNAs are being investigated to silence genes contributing to diseases like Huntington’s disease or Alzheimer’s disease.
Ocular Diseases: siRNA therapies targeting angiogenesis-related genes (e.g., VEGF) are being developed for age-related macular degeneration.
5. Design Considerations for siRNAs
Effective siRNA design requires careful consideration to maximize specificity, potency, and stability:
Sequence Specificity: The guide strand must perfectly complement the target mRNA to minimize off-target effects. Regions prone to secondary structures (e.g., hairpins) in the mRNA should be avoided for targeting.
Thermodynamic Stability: The duplex should have lower thermodynamic stability at the 5’ end of the guide strand to favor its incorporation into RISC.
Chemical Modifications: siRNAs are prone to degradation by nucleases and can trigger immune responses. Modifications improve their pharmacokinetics: 2’-O-methyl or 2’-fluoro modifications on sugar rings increase stability. Phosphorothioate backbone modifications enhance nuclease resistance.
Avoiding Off-Target Effects: siRNAs can sometimes bind unintended mRNAs with partial complementarity. Proper design minimizes this risk by: Avoiding homology to unrelated genes. Performing genome-wide alignment checks.
Delivery Challenges: Efficient delivery to target tissues remains a major hurdle. Delivery strategies include: Lipid nanoparticles (e.g., for liver targeting). Conjugation to GalNAc (N-acetylgalactosamine) for hepatocyte-specific delivery.
6. Comparison to Other RNA-Based Therapeutics
siRNAs are part of a broader family of RNA-targeting tools. How they compare:
vs. Antisense Oligonucleotides (ASOs): ASOs are single-stranded and often degrade RNA via RNase H or steric blockade, whereas siRNAs use the RISC complex for mRNA cleavage. siRNAs are more efficient due to RISC recycling but are more complex to deliver.
vs. miRNAs: siRNAs are synthetic and designed to target one specific mRNA, while miRNAs are endogenous and can regulate multiple targets.
Challenges and Future Directions
Delivery Barriers: Systemic delivery often leads to uptake in the liver; targeting other tissues (e.g., brain, muscle) remains a challenge.
Off-Target Effects: Improved design algorithms are being developed to minimize unintended interactions.
Immune Activation: Modifications continue to refine siRNAs to avoid triggering innate immunity (e.g., Toll-like receptor activation).
Expanding Applications: Beyond gene silencing, siRNAs are being adapted for gene editing and other novel applications.
microRNAs (miRNAs)
These fascinating molecules are critical regulators of gene expression in eukaryotic cells and play pivotal roles in development, physiology, and disease.
1. What Are miRNAs?
miRNAs are endogenous, small, non-coding RNAs, typically about 20-24 nucleotides in length, that regulate gene expression post-transcriptionally. They achieve this by binding to complementary sequences within the 3' untranslated regions (UTRs) of target mRNAs, leading to:
Translation repression (blocking protein production).
mRNA degradation (reducing transcript levels).
Think of miRNAs as gene expression moderators, akin to dimmer switches that fine-tune protein production.
2. miRNA Biogenesis (How Are miRNAs Made?)
The production of miRNAs involves a multi-step process in both the nucleus and the cytoplasm:
Step 1: Transcription of miRNA Genes
miRNAs are encoded in the genome and transcribed by RNA polymerase II or RNA polymerase III as part of primary miRNA transcripts (pri-miRNAs).
Pri-miRNAs are often several kilobases long and contain one or more hairpin structures (precursors to mature miRNAs).
Step 2: Processing by Drosha and DGCR8
In the nucleus, pri-miRNAs are processed by the Microprocessor Complex, consisting of: Drosha: An RNase III enzyme that cleaves the pri-miRNA. DGCR8: A double-stranded RNA-binding protein that stabilizes pri-miRNAs.
This processing generates a precursor miRNA (pre-miRNA), which is a short hairpin RNA (~70 nucleotides).
Step 3: Export to the Cytoplasm
The Exportin-5 protein transports the pre-miRNA from the nucleus to the cytoplasm in a Ran-GTP–dependent manner.
Step 4: Processing by Dicer
In the cytoplasm, the pre-miRNA is further processed by Dicer, another RNase III enzyme, which removes the loop of the hairpin structure to produce a double-stranded miRNA duplex (~22 base pairs).
Step 5: Incorporation into RISC
One strand of the miRNA duplex (the guide strand) is loaded into the RNA-induced silencing complex (RISC), which includes: Argonaute (AGO) proteins: AGO2 is the key catalytic component.
The other strand (the passenger strand) is degraded.
3. How Do miRNAs Regulate Gene Expression?
miRNAs guide RISC to target mRNAs based on complementarity between the miRNA and the mRNA’s 3' UTR. The outcome depends on the degree of base-pairing:
Perfect or Near-Perfect Complementarity:
Common in plants.
The target mRNA is cleaved by AGO2, leading to degradation and a complete halt of protein production.
Partial Complementarity:
Common in animals.
The miRNA-mRNA interaction results in: Translation Repression: Ribosomes are prevented from translating the mRNA into protein. mRNA Destabilization: Accelerated deadenylation (removal of the poly-A tail) and decapping, followed by mRNA degradation.
4. miRNA Targeting Specificity
miRNA targeting is governed by:
Seed Sequence: A critical region spanning nucleotides 2-7 at the 5’ end of the miRNA. Perfect complementarity in the seed region is essential for strong binding.
Target Site Context: The surrounding mRNA sequence and secondary structure can influence binding.
Multiple Targets: A single miRNA can regulate hundreds of mRNAs, making miRNAs powerful regulators of gene networks.
Cooperative Regulation: Multiple miRNAs can bind different sites on the same mRNA for additive or synergistic effects.
5. Functions of miRNAs
miRNAs are involved in nearly every cellular process by regulating gene expression networks. Here are some key roles:
A. Development and Differentiation
miRNAs control cell fate decisions, tissue development, and differentiation. Example: miR-1 regulates muscle cell differentiation.
B. Homeostasis
miRNAs help maintain cellular balance by fine-tuning processes like apoptosis, proliferation, and metabolism. Example: miR-21 modulates cell survival pathways in response to stress.
C. Immune Response
miRNAs regulate immune signaling and inflammation. Example: miR-155 enhances inflammatory cytokine production.
D. Cancer
miRNAs act as either oncogenes (oncomiRs) or tumor suppressors: OncomiRs: Promote cancer by downregulating tumor suppressor genes (e.g., miR-21). Tumor-Suppressive miRNAs: Prevent cancer by silencing oncogenes (e.g., let-7).
E. Neurological Disorders
Dysregulation of miRNAs is linked to neurodegenerative diseases, such as Alzheimer’s and Parkinson’s. Example: miR-124 is essential for neuronal differentiation.
6. Therapeutic Applications of miRNAs
Harnessing miRNAs for therapeutic purposes is a growing area of research:
A. miRNA Mimics
Synthetic miRNAs are designed to restore the function of downregulated miRNAs. Example: Using miRNA mimics to suppress oncogene expression in cancer.
B. Anti-miRs (miRNA Inhibitors)
Chemically modified oligonucleotides (e.g., antagomirs or locked nucleic acids [LNAs]) are used to inhibit overactive miRNAs. Example: Targeting miR-122 to treat hepatitis C virus (HCV) infection.
C. miRNA Biomarkers
Changes in miRNA expression are used as diagnostic or prognostic markers for diseases. Example: Circulating miRNAs in blood can indicate cancer progression or cardiovascular health.
7. Challenges in miRNA Therapeutics
Delivery: Efficiently delivering miRNA-based therapies to specific tissues remains a major hurdle. Delivery systems include lipid nanoparticles, viral vectors, or conjugated oligonucleotides (e.g., GalNAc for liver targeting).
Off-Target Effects: miRNAs often target multiple genes, increasing the risk of unintended effects.
Stability: Unmodified miRNAs are rapidly degraded in vivo, requiring chemical stabilization.
Immune Activation: Exogenous miRNAs can trigger immune responses through Toll-like receptors (TLRs).
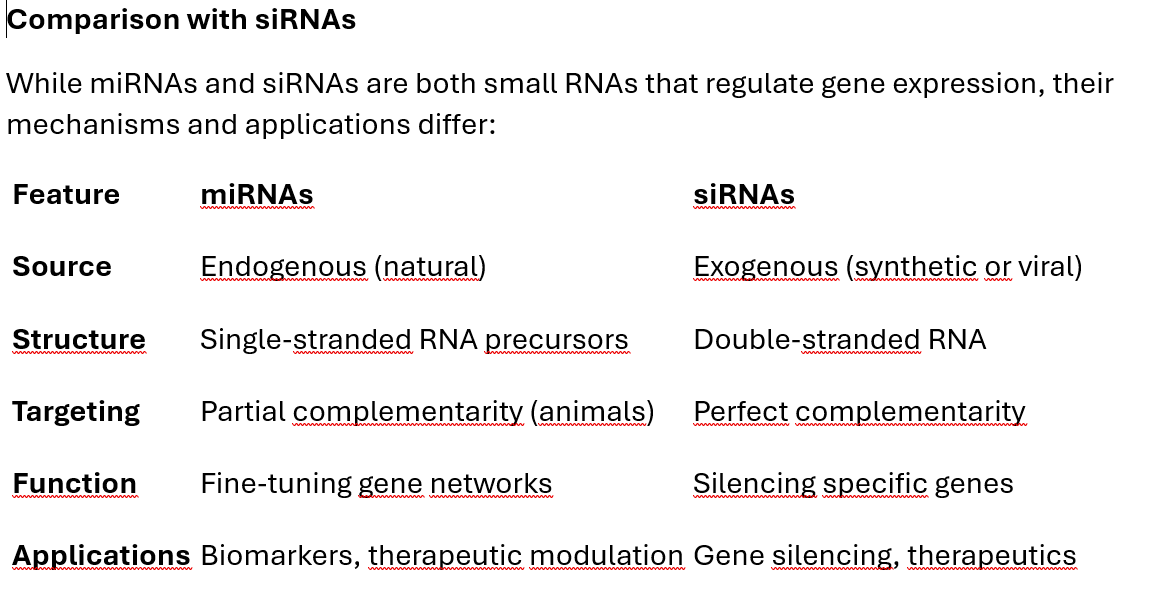
8. Comparison with siRNAs
While miRNAs and siRNAs are both small RNAs that regulate gene expression, their mechanisms and applications differ:
RNA Aptamers: A Detailed Exploration
RNA aptamers are short, single-stranded RNA (or DNA) sequences that fold into unique three-dimensional structures, enabling them to bind specifically to target molecules. Their ability to interact with proteins, small molecules, or even entire cells makes them functionally similar to antibodies. However, their small size, synthetic nature, and tunable design provide several advantages over antibodies in therapeutic and diagnostic applications.
1. What Are RNA Aptamers?
An RNA aptamer is essentially a "molecular key" designed to fit a specific "lock" (a target molecule). These RNA sequences form complex secondary and tertiary structures, such as:
Hairpins
Loops
Bulges
Pseudoknots
These structural elements create binding sites with high affinity (strength of binding) and specificity (selectivity for the target).
2. How Are RNA Aptamers Made?
RNA aptamers are typically selected through a process called Systematic Evolution of Ligands by Exponential Enrichment (SELEX). This iterative method refines a pool of random RNA sequences to identify high-affinity binders for a specific target.
Step 1: Random Library Generation
A large, diverse pool of single-stranded RNA sequences is synthesized. These sequences are typically 40-100 nucleotides long and contain random regions flanked by constant primer-binding regions.
Step 2: Binding to Target
The RNA pool is incubated with the target molecule (e.g., a protein).
RNAs that bind the target are retained, while unbound sequences are washed away.
Step 3: Amplification
Bound RNA sequences are reverse transcribed into complementary DNA (cDNA) using reverse transcriptase.
The cDNA is amplified via PCR to regenerate the RNA library for the next round of selection.
Step 4: Iterative Selection
This process is repeated (usually 8–15 rounds), progressively enriching the pool for high-affinity binders.
Step 5: Cloning and Sequencing
After the final round, the enriched sequences are cloned, sequenced, and analyzed to identify the most effective aptamers.
3. How Do RNA Aptamers Work?
RNA aptamers function by binding to their targets through specific non-covalent interactions, such as:
Hydrogen bonds
Van der Waals forces
Electrostatic interactions
The specificity comes from the aptamer's ability to fold into a shape complementary to the target's surface or active site. Once bound, aptamers can:
Inhibit the target's function by blocking active sites or interaction domains (e.g., preventing enzyme activity or ligand binding).
Act as molecular sensors by binding and signaling the presence of their target.
4. Applications of RNA Aptamers
RNA aptamers are versatile tools in both research and therapeutics. Their applications can be categorized as follows:
A. Therapeutics
Inhibitors: RNA aptamers can block the activity of disease-related proteins, functioning as antagonists. Example: Pegaptanib (Macugen), an FDA-approved RNA aptamer, inhibits vascular endothelial growth factor (VEGF) to treat age-related macular degeneration (AMD).
Drug Delivery: Aptamers can act as targeting ligands for delivering drugs to specific cells or tissues. Example: Aptamers conjugated to nanoparticles for targeted cancer therapy.
Anticoagulants: Aptamers targeting clotting factors (e.g., thrombin) are being developed as reversible anticoagulants.
B. Diagnostics
RNA aptamers can be used as biosensors due to their ability to bind specific biomarkers with high affinity.
Example: Aptamer-based sensors to detect small molecules like glucose or proteins like cardiac troponin for heart attack diagnosis.
C. Research Tools
Protein Characterization: Aptamers can isolate and study specific proteins by acting as "molecular handles."
Imaging: Fluorophore-labeled aptamers are used for visualizing target molecules in live cells or tissues.
5. Advantages of RNA Aptamers
RNA aptamers have unique properties that make them attractive alternatives to antibodies:
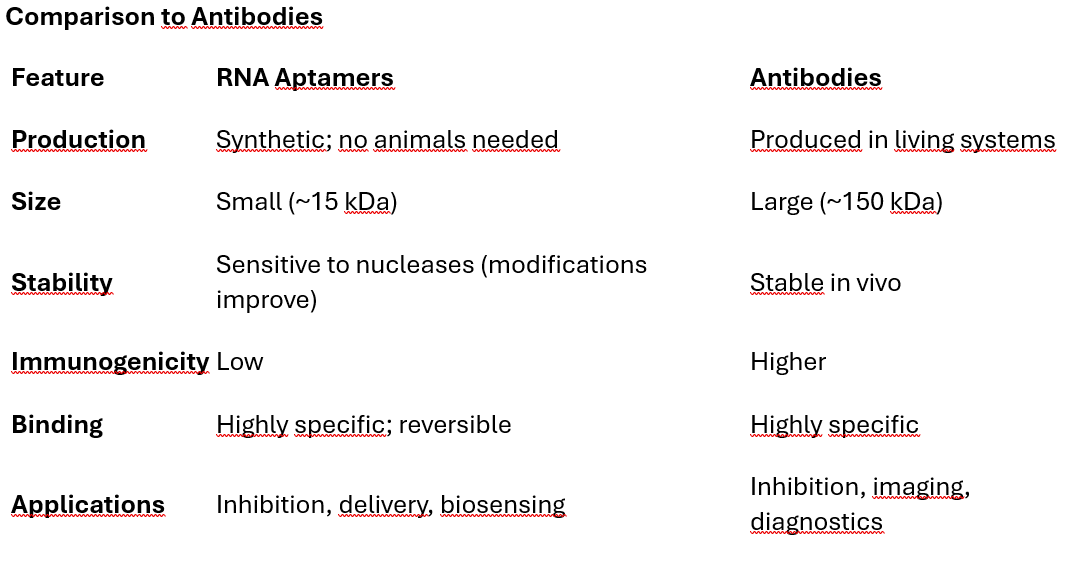
Synthetic and Chemical Nature: Aptamers are synthesized chemically, ensuring batch-to-batch consistency. No reliance on living organisms for production (unlike antibodies).
Small Size: Aptamers are smaller than antibodies (~15 kDa vs. ~150 kDa), allowing better tissue penetration.
Low Immunogenicity: Aptamers, being non-proteinaceous, are less likely to trigger an immune response.
Reversible Binding: Some aptamers can be "turned off" by introducing complementary oligonucleotides, enabling control over their activity.
Broad Target Range: Aptamers can bind to a wide variety of molecules, from small ions to large proteins, with high specificity.
6. Challenges in Using RNA Aptamers
Despite their advantages, RNA aptamers face several challenges:
Stability: RNA aptamers are susceptible to degradation by nucleases in biological fluids. Solution: Chemical modifications like 2'-fluoro or 2'-O-methyl groups increase nuclease resistance.
Short Half-Life: Without modifications, aptamers are rapidly cleared from circulation. Solution: PEGylation (attachment of polyethylene glycol) extends circulation time.
Delivery: Efficiently delivering aptamers to specific tissues or across cellular membranes remains a major hurdle.
Cost: The SELEX process and chemical synthesis can be expensive, especially for large-scale applications.
Comparison to Antibodies
8. Future Directions for RNA Aptamers
Engineering Super-Stable Aptamers: Incorporation of unnatural nucleotides or locked nucleic acids (LNAs) to further enhance stability.
Targeting Intracellular Proteins: Developing strategies to deliver aptamers into cells for targeting intracellular proteins.
Aptamer-Based Drug Conjugates: Linking aptamers with drugs or toxins for targeted cancer therapy.
Integration with CRISPR: Using aptamers as programmable guides for CRISPR-based gene editing.
Ribozymes: A Technical Deep Dive
Ribozymes, or RNA enzymes, are RNA molecules with catalytic activity. Unlike typical RNAs that primarily serve as carriers of genetic information, ribozymes are capable of catalyzing specific biochemical reactions, such as RNA cleavage and ligation. These remarkable molecules play important roles in natural cellular processes and hold potential for therapeutic and biotechnological applications.
Let’s break down their structure, mechanism of action, types, and applications in detail.
1. What Are Ribozymes?
A ribozyme is a single-stranded RNA molecule that folds into a specific three-dimensional structure, enabling it to catalyze a chemical reaction. The catalytic activity of ribozymes arises from their ability to:
Form complex secondary and tertiary structures (e.g., stem-loops, pseudoknots).
Coordinate metal ions (e.g., Mg²⁺) that stabilize the transition state or participate in catalysis.
Key functions of ribozymes include:
Cleavage of phosphodiester bonds in RNA.
Ligation of RNA fragments.
Peptide bond formation (as in the ribosome).
2. Ribozymes in Nature
Ribozymes occur naturally in all domains of life and play vital roles in RNA processing and gene regulation. Examples include:
A. Self-Cleaving Ribozymes
These ribozymes cleave themselves out of a longer RNA precursor.
Example: Hammerhead ribozyme (common in viroids and satellite RNAs of plants).
B. Catalysts in Ribosomal Function
The ribosome itself is a ribozyme, where the 23S rRNA (in prokaryotes) or 28S rRNA (in eukaryotes) catalyzes peptide bond formation during protein synthesis.
C. RNA Splicing
The group I and group II introns are ribozymes that catalyze their own excision from precursor RNAs and ligate the remaining exons.
3. How Do Ribozymes Work?
The catalytic activity of ribozymes involves acid-base catalysis, nucleophilic attack, and transition state stabilization. Here’s a detailed look at their mechanism:
Step 1: RNA Folding
Ribozymes fold into a precise three-dimensional structure through:
Base pairing (secondary structure).
Long-range interactions like pseudoknots and metal ion coordination (tertiary structure).
Step 2: Substrate Binding
Ribozymes recognize their RNA substrate through Watson-Crick base pairing or structural complementarity.
Step 3: Cleavage Reaction
The catalytic reaction typically involves:
Activation of the 2’-OH group: The ribozyme's structure or a bound metal ion (e.g., Mg²⁺) deprotonates the substrate’s 2’-OH group, making it a nucleophile.
Nucleophilic Attack: The activated 2’-OH group attacks the phosphate group at the 3’ position of an adjacent nucleotide, breaking the phosphodiester bond.
Leaving Group Formation: This reaction forms a cyclic 2’,3’-phosphate at the cleavage site and a free 5’-hydroxyl group.
4. Types of Ribozymes
There are several classes of ribozymes based on their natural function or engineered design:
A. Small Self-Cleaving Ribozymes
Hammerhead Ribozyme: Found in plant viroids and satellite RNAs. Cleaves its own RNA backbone via a single turnover mechanism.
Hepatitis Delta Virus (HDV) Ribozyme: Found in the hepatitis delta virus. Performs a self-cleavage reaction required for viral replication.
Hairpin Ribozyme: Found in satellite RNAs of plant viruses. Requires divalent cations (e.g., Mg²⁺) for catalysis.
B. Large Catalytic Ribozymes
Group I Introns: Catalyze self-splicing by using an external guanosine molecule as a cofactor. Found in rRNA, mRNA, and tRNA genes of certain organisms.
Group II Introns: Catalyze self-splicing through a lariat intermediate, similar to the spliceosome mechanism.
C. Engineered Ribozymes
Artificial Ribozymes: Designed through in vitro evolution to cleave specific RNA sequences (e.g., using SELEX). Example: "RNA-cleaving DNAzymes" or customized ribozymes for therapeutics.
Ribozyme Therapeutics: Engineered ribozymes target specific mRNA sequences to silence gene expression.
5. Applications of Ribozymes
A. Therapeutics
Ribozymes are being explored for gene-silencing applications, especially for diseases caused by overactive or defective genes.
Targeted mRNA Cleavage: Ribozymes can be designed to bind and cleave specific mRNAs, reducing the production of disease-causing proteins. Example: Ribozymes targeting viral RNA in HIV or hepatitis B.
Cancer Therapy: Engineered ribozymes can cleave oncogenic mRNAs or those associated with drug resistance.
Neurological Disorders: Ribozymes targeting RNA implicated in diseases like Huntington’s or Alzheimer’s.
B. Molecular Biology Tools
Gene Knockdown: Ribozymes are used to selectively knock down specific genes in research.
RNA Manipulation: Ribozymes are used to modify RNA sequences for studying RNA structure and function.
C. Synthetic Biology
Ribozymes are incorporated into genetic circuits for:
Biosensing: Detecting specific RNA or chemical molecules.
Toggle switches: Controlling gene expression in response to environmental signals.
6. Advantages of Ribozymes
Specificity: Ribozymes can be designed to cleave a single mRNA sequence, minimizing off-target effects.
Catalytic Efficiency: Ribozymes can cleave multiple RNA molecules in succession, functioning as true enzymes.
Synthetic Production: Unlike proteins, ribozymes can be easily synthesized and engineered.
7. Challenges of Ribozymes
Stability: Natural ribozymes are sensitive to degradation by ribonucleases (RNases). Solution: Chemical modifications, such as 2’-fluoro or locked nucleic acids (LNAs), enhance stability.
Delivery: Efficiently delivering ribozymes to target tissues and cells remains a challenge. Solution: Delivery via viral vectors, lipid nanoparticles, or aptamer conjugation.
Limited Targets: Ribozymes require accessible single-stranded regions in the target RNA.
8. Examples of Ribozyme-Based Therapeutics
Angiozyme: A ribozyme targeting VEGF receptor mRNA, investigated for anti-cancer therapy.
Heptazyme: A ribozyme targeting hepatitis C virus (HCV) RNA.
TAT-Rz: A ribozyme designed to cleave HIV tat transcripts.
9. Future Directions
Ribozymes in CRISPR Systems: Coupling ribozymes with CRISPR tools for programmable RNA manipulation.
Synthetic Ribozyme Engineering: Creating ribozymes for new catalytic reactions beyond RNA cleavage.
Integration with Nanotechnology: Combining ribozymes with nanoparticles for more effective delivery and stability.
mRNA Therapeutics: A Comprehensive Exploration
mRNA therapeutics represent a groundbreaking approach in medicine, enabling the in vivo production of therapeutic proteins by delivering synthetic messenger RNA (mRNA) to cells. These therapies are highly versatile, with applications ranging from vaccines to enzyme replacement therapy and cancer immunotherapy.
1. What Are mRNA Therapeutics?
mRNA therapeutics involve the use of synthetic, modified mRNA molecules to direct the cellular machinery to produce specific proteins. The therapeutic effect is achieved by:
Introducing the synthetic mRNA into target cells.
Allowing the mRNA to be translated by ribosomes into functional proteins.
Key Advantages:
Non-permanent: Unlike DNA-based approaches, mRNA does not integrate into the genome, reducing long-term risks.
Rapid Development: Synthetic mRNA can be designed and produced quickly, making it ideal for responding to emerging diseases.
Versatility: Any protein of interest can, in principle, be encoded and delivered.
2. How Are mRNA Therapeutics Made?
mRNA therapeutics are carefully engineered to optimize their stability, translatability, and safety. The process involves multiple steps:
Step 1: Template Design
A DNA template encoding the desired protein is designed with the following components: Cap structure: Enhances mRNA stability and translation initiation. 5' Untranslated Region (UTR): Optimized to regulate translation efficiency. Coding sequence (CDS): Specifies the amino acid sequence of the therapeutic protein. 3' Untranslated Region (UTR): Affects mRNA stability and translational efficiency. Poly(A) tail: Protects the mRNA from degradation and enhances translation.
Step 2: In Vitro Transcription (IVT)
Using the DNA template, synthetic mRNA is transcribed in vitro using a phage RNA polymerase (e.g., T7, SP6).
The IVT process produces linear mRNA with modifications that enhance its stability and minimize immune activation.
Step 3: Modifications
To improve the stability and functionality of the mRNA, several chemical modifications are introduced:
Cap Analogues: A synthetic 5’-cap structure is added enzymatically or during IVT to mimic natural mRNA.
Modified Nucleosides: Substituting uridine with N1-methyl-pseudouridine or other analogues reduces immune recognition and improves stability.
Poly(A) Tail Optimization: The length of the tail (~100-150 adenosines) is carefully controlled to balance stability and translation efficiency.
Step 4: Purification
The mRNA is purified to remove impurities, such as double-stranded RNA (dsRNA), which can trigger unwanted immune responses.
Step 5: Delivery Vehicle Development
Naked mRNA is unstable and cannot efficiently enter cells, so it is encapsulated in a delivery vehicle, such as lipid nanoparticles (LNPs).
3. Mechanism of Action
The process of delivering and expressing mRNA in target cells involves several steps:
Step 1: Delivery
The mRNA is packaged in lipid nanoparticles or other carriers to: Protect it from degradation by nucleases in the bloodstream. Facilitate cellular uptake through endocytosis.
Step 2: Endosomal Escape
Once inside the cell, the nanoparticles are taken up by endosomes.
Lipid components of the nanoparticle destabilize the endosomal membrane, releasing the mRNA into the cytoplasm.
Step 3: Translation
In the cytoplasm, the mRNA is translated by ribosomes into the encoded protein.
The translated protein can: Act as a vaccine antigen (e.g., spike protein in COVID-19 vaccines). Replace a missing or defective protein in genetic disorders.
Step 4: Degradation
The synthetic mRNA is eventually degraded by natural RNA turnover pathways, ensuring no long-term persistence.
4. Applications of mRNA Therapeutics
A. mRNA Vaccines
Mechanism: Encoded antigens (e.g., viral spike proteins) are translated and presented on cell surfaces to activate the immune system.
Example: COVID-19 vaccines (Pfizer-BioNTech and Moderna). Encodes the SARS-CoV-2 spike protein. Induces robust humoral (antibody) and cellular (T-cell) responses.
B. Protein Replacement Therapy
Used to treat genetic diseases caused by a lack of functional proteins.
Example: Cystic fibrosis: mRNA encodes functional CFTR protein to replace defective versions.
C. Cancer Immunotherapy
Mechanism: Encodes tumor-specific antigens or immune modulators to stimulate the immune system against cancer cells.
Example: mRNA encoding neoantigens (mutated proteins specific to a tumor).
D. Regenerative Medicine
mRNA therapeutics can drive the production of growth factors or other regenerative proteins in damaged tissues.
E. Infectious Disease
Beyond COVID-19, mRNA vaccines are being developed for other pathogens like influenza, HIV, Zika, and rabies.
5. Advantages of mRNA Therapeutics
Rapid Development: mRNA vaccines for COVID-19 were developed in record time due to the platform’s flexibility.
No Risk of Integration: Unlike DNA-based therapies, mRNA does not integrate into the genome, avoiding mutagenesis risks.
Customizability: mRNA can be designed to encode any protein, enabling personalized therapies.
Scalable Manufacturing: Synthetic mRNA production is efficient and does not require cell cultures.
6. Challenges of mRNA Therapeutics
Despite their promise, several challenges remain:
A. Delivery
Efficient delivery to target tissues and cells is critical.
Current solutions: Lipid Nanoparticles (LNPs): Encapsulate mRNA for delivery, but targeting specific tissues (e.g., brain) remains a challenge.
B. Stability
Unmodified mRNA is inherently unstable and prone to degradation by RNases.
Solution: Incorporating modified nucleosides and optimized storage conditions.
C. Immunogenicity
Synthetic mRNA can trigger innate immune responses via pattern recognition receptors (e.g., Toll-like receptors).
Solution: Modifying mRNA with pseudouridine or removing dsRNA contaminants.
D. Cost
mRNA therapeutics are expensive to produce and require cold-chain storage for stability.
7. Future Directions
Self-Amplifying mRNA (saRNA): Includes sequences encoding an RNA-dependent RNA polymerase (RdRP) to amplify itself after delivery, reducing dosage requirements.
Targeted Delivery: Developing carriers for specific tissues, such as GalNAc-conjugated nanoparticles for liver targeting.
Thermostable Formulations: Improving mRNA stability at room temperature to simplify distribution logistics.
Expanded Disease Targets: Exploring applications in rare diseases, autoimmune disorders, and neurodegenerative conditions.
Conclusion
Nucleic acid-based therapeutics have ushered in a transformative era in medicine, redefining how we approach disease treatment by targeting its genetic roots. These advanced therapies leverage the unique properties of DNA and RNA to modulate gene expression with unparalleled precision, offering new hope for conditions ranging from rare genetic disorders to widespread challenges like cancer and infectious diseases. As the field continues to mature, these therapeutics promise not only to enhance patient outcomes but also to reshape our broader understanding of disease management, shifting the focus from generalized approaches to highly tailored, molecular-level interventions.
The future of this technology is marked by incredible potential. Emerging innovations in delivery systems, such as lipid nanoparticles and tissue-specific targeting strategies, are addressing longstanding challenges of stability and precision, while chemical modifications are further enhancing the safety and efficacy of nucleic acid molecules. Advances in mRNA therapeutics, already demonstrated in the success of COVID-19 vaccines, are paving the way for rapid responses to emerging diseases and new applications in cancer immunotherapy and protein replacement therapies. Meanwhile, the rise of personalized medicine, fueled by breakthroughs in genomic sequencing, is enabling the development of therapies tailored to the unique genetic makeup of individual patients. This synergy between cutting-edge molecular biology and clinical innovation heralds a future where treatments are not only more effective but also more inclusive, addressing a broader spectrum of diseases.
As we look ahead, the continued evolution of nucleic acid-based therapeutics holds profound implications for healthcare. The integration of artificial intelligence and bioinformatics into therapeutic design, along with advances in synthetic biology and nanotechnology, promises to refine and expand the scope of these treatments. Challenges remain, particularly in ensuring equitable access, reducing production costs, and addressing regulatory hurdles, but the trajectory of the field is unmistakable. With ongoing interdisciplinary collaboration and investment, nucleic acid-based therapies are poised to fulfill their transformative potential, offering new paradigms of treatment and a glimpse into a future where we can target diseases at their very source with precision, efficiency, and hope.
#NucleicAcidTherapeutics #GeneSilencing #OligonucleotideTherapeutics #mRNATherapeutics #RNAInterference #AntisenseOligonucleotides #GeneExpressionModulation #RNAAptamers #TargetedTherapies #PersonalizedMedicine