What Is Oligonucleotide Synthesis? Phosphoramidite oligonucleotide synthesis
Learn how scientists create custom DNA and RNA strands for use in research, diagnostics, and drug development.
Oligonucleotide synthesis represents a foundational technique in molecular biology, biotechnology, and genomics, enabling the creation of custom-designed short sequences of nucleotides—DNA or RNA fragments—crucial for numerous applications such as genetic testing, diagnostics, gene editing, synthetic biology, and therapeutic development. The ability to synthesize these molecules with high specificity and precision has revolutionized the study of nucleic acids and facilitated a broad range of innovations across multiple scientific disciplines. Typically ranging between 5 and 100 nucleotides in length, oligonucleotides serve as primers for PCR, molecular probes, antisense therapies, and components in gene assembly, underscoring their indispensable role in both experimental and applied molecular sciences.
Overview of Oligonucleotide Synthesis Methods
1. Phosphoramidite Synthesis (Solid-Phase)
Gold standard method for synthesizing high-purity, custom ssDNA and RNA.
Involves stepwise addition of nucleoside phosphoramidites on a solid support (e.g., CPG or polystyrene).
Utilizes protecting groups (e.g., DMT) and a cycle of deprotection, coupling, capping, and oxidation.
Ideal for synthesizing oligos up to ~150–200 nucleotides.
2. Microarray-Based Synthesis
Enables high-throughput production of thousands to millions of oligos in parallel on a single chip.
Uses either photolithography (light-directed synthesis with photomasks) or inkjet printing (spot-by-spot deposition of reagents).
Common in genomics, gene expression profiling, and CRISPR screening.
Produces shorter sequences (typically <100 nucleotides) due to lower coupling efficiency.
3. PCR-Based Synthesis
Primarily used to amplify and modify DNA fragments.
Employs synthetic primers and DNA polymerase for exponential replication.
Variants include:
Overlap Extension PCR: Joins DNA fragments with complementary ends.
Polymerase Cycling Assembly (PCA): Builds long sequences from short overlapping oligos.
Useful for assembling genes and mutagenesis.
4. Enzymatic DNA Synthesis
An emerging, biological alternative to chemical synthesis.
Uses enzymes like DNA polymerases to extend DNA strands with high fidelity and potential for longer sequences.
Key techniques:
Terminal Deoxynucleotidyl Transferase (TdT) Synthesis: Adds nucleotides to 3' ends without a template—ideal for labeling or randomized sequences.
Rolling Circle Amplification (RCA): Amplifies circular DNA templates continuously; great for diagnostics and nanotech applications.
5. Gene Synthesis via Oligo Assembly
Constructs entire genes from overlapping oligonucleotide fragments.
Common methods include:
Gibson Assembly: Uses exonucleases, polymerase, and ligase to seamlessly join fragments.
Golden Gate Assembly: Leverages Type IIS restriction enzymes for scarless assembly.
SLIC (Sequence and Ligation Independent Cloning): Uses homologous recombination and minimal enzymes.
Essential for synthetic biology, metabolic engineering, and custom genetic constructs.
As this article series unfolds, we’ll explore each method in detail—covering workflows, reagents, advantages, limitations, and real-world applications across biotech, pharma, and academic research. This first article will cover method 1.
1. Phosphoramidite oligonucleotide synthesis
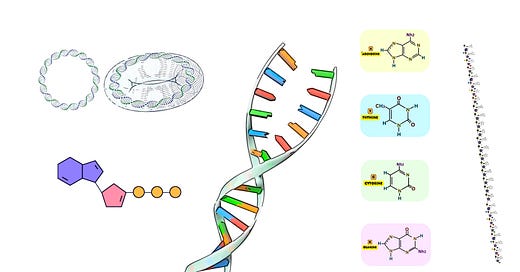
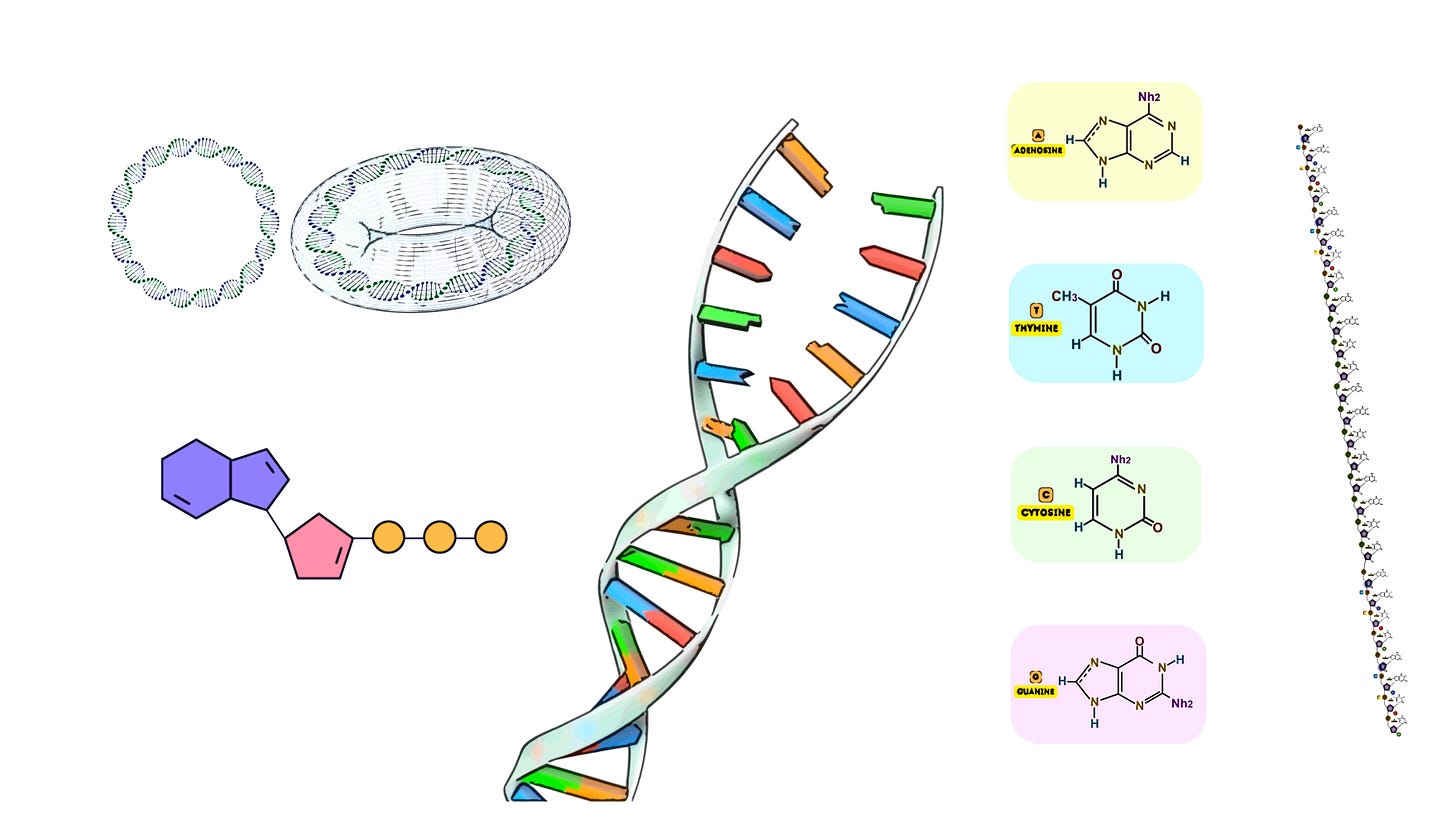
The phosphoramidite method is the most widely used and established chemical process for synthesizing short DNA sequences known as oligonucleotides. This technique is based on solid-phase chemistry, and its reliability and high efficiency have made it the gold standard for generating oligonucleotides up to around 200 nucleotides long.
At the core of the phosphoramidite method is a stepwise addition of nucleotide monomers to a growing oligonucleotide chain, each protected by a phosphoramidite group. The process ensures high fidelity, enabling the synthesis of sequences with exact nucleotide compositions. Let’s dive into the technical details of each stage of this method.
Key Components of the Phosphoramidite Method
Before we break down the synthesis process, it's essential to understand the core components used in this method:
Phosphoramidite Nucleotides: These are the building blocks of DNA synthesis. Each phosphoramidite is a chemically protected version of a nucleotide, with protection groups attached to the 5’-hydroxyl group and the phosphate backbone to control reactivity during the synthesis process.
Solid Support: The process begins with an immobilized starting nucleotide or a nucleoside attached to a solid support, such as controlled pore glass (CPG) or polystyrene beads. This solid support allows the growing oligonucleotide chain to be retained throughout the synthesis, while excess reagents can be easily washed away.
Protecting Groups:
DMT (Dimethoxytrityl): The 5’-OH group of each nucleotide is protected by a DMT group, preventing unwanted side reactions during synthesis.
Cyanoethyl protecting group: The 3’ phosphate group is protected by a β-cyanoethyl group, stabilizing the phosphate linkage as it forms.
Activators: Tetrazole derivatives are used as activators to convert the phosphoramidite into a more reactive form, enabling the nucleotide addition reaction.
Step-by-Step Process of Phosphoramidite Oligonucleotide Synthesis
The process of synthesizing oligonucleotides using the phosphoramidite method consists of a cyclic, four-step process for each nucleotide addition. This cycle is repeated until the entire desired sequence is assembled.
Deprotection (Removal of the DMT Group)
The first step of the synthesis cycle is deprotection, where the 5'-DMT group on the terminal nucleotide is removed, exposing the reactive 5'-hydroxyl group (–OH) that will participate in the next nucleotide coupling reaction.
The deprotection is accomplished using trichloroacetic acid (TCA) or dichloroacetic acid (DCA) in an organic solvent such as dichloromethane.
The DMT group, being hydrophobic, turns the solution orange upon its removal, providing a visual indicator that the reaction has proceeded correctly.
This step ensures that the growing DNA chain is primed to accept the next nucleotide in the sequence.
Coupling (Addition of Phosphoramidite Nucleotide)
The coupling step is the heart of the synthesis process. In this step, a new phosphoramidite nucleotide is added to the growing oligonucleotide chain.
The free 5'-hydroxyl group of the terminal nucleotide reacts with the incoming 3'-phosphoramidite group of the next nucleotide, forming a phosphite triester bond between the two nucleotides.
The coupling reaction is typically catalyzed by an activator such as tetrazole or ethylthiotetrazole, which makes the phosphoramidite more reactive by forming a highly reactive tetrazolylphosphite intermediate.
The efficiency of the coupling step is crucial for maintaining high synthesis yield. Typically, the efficiency of each coupling reaction exceeds 99%, but small inefficiencies accumulate over many cycles, limiting the length of high-fidelity oligonucleotides to around 200 nucleotides.
Capping (Termination of Unreacted Chains)
Not every nucleotide coupling step will be 100% efficient, meaning that some of the growing oligonucleotide chains might not receive the next nucleotide. If these chains were left intact, they could continue to participate in subsequent coupling steps, leading to deletion mutants or incomplete sequences.
To prevent this, a capping step is employed after each coupling reaction:
Unreacted 5’-hydroxyl groups are acetylated using a mixture of acetic anhydride and N-methylimidazole. This reaction covalently caps the unreacted oligonucleotide chains, preventing them from reacting in subsequent steps.
Capping ensures that only the correctly elongated chains continue in the synthesis process, thus improving the overall purity of the final product.
Oxidation (Stabilization of the Phosphate Linkage)
After the coupling reaction, the bond between the newly added nucleotide and the growing chain is still in the form of a phosphite triester, which is chemically unstable. To convert this to the more stable phosphate triester, an oxidation step is performed:
The phosphite triester is oxidized using an iodine solution (often in a mixture of water, pyridine, and tetrahydrofuran (THF)).
This oxidation converts the phosphite triester into a phosphate diester, stabilizing the backbone of the oligonucleotide and completing the addition of the nucleotide.
Final Cleavage and Deprotection
Once the full oligonucleotide sequence has been assembled, the synthesis must be terminated and the oligonucleotide released from the solid support. This involves several steps:
Cleavage from the Solid Support: The oligonucleotide is cleaved from the solid support using a basic solution, such as concentrated ammonium hydroxide. This breaks the ester bond linking the first nucleotide to the solid support.
Removal of Protecting Groups: The cyanoethyl protecting groups on the phosphate backbone are removed under basic conditions, often at the same time as the cleavage from the support. This restores the free phosphate groups along the backbone.
Purification: The crude oligonucleotide mixture is then purified, typically by high-performance liquid chromatography (HPLC) or polyacrylamide gel electrophoresis (PAGE), to separate the full-length product from truncated sequences or byproducts.
Desalting: The final oligonucleotide is desalted to remove any remaining salts, reagents, or small molecules from the synthesis process.
Key Considerations and Challenges
Although the phosphoramidite method is highly efficient and well-suited for the synthesis of oligonucleotides, there are a few critical considerations and challenges to bear in mind:
Yield and Length Limitations:
The efficiency of each step in the synthesis process is high (>99%), but as the sequence length increases, cumulative inefficiencies become significant.
For instance, if each coupling step is 99% efficient, then the overall yield of a 100-mer oligonucleotide would be around 37% (0.99^100), meaning that the fraction of full-length products decreases with sequence length.
Synthesis Time:
The phosphoramidite synthesis method is generally rapid, but the time required increases with the length of the oligonucleotide due to the repetitive nature of the process.
Automated synthesizers can typically synthesize an oligonucleotide of about 20 nucleotides in a matter of hours, but longer sequences (100-200 bases) may take significantly longer, especially when factoring in purification.
Chemical Purity:
Purification steps such as HPLC or PAGE are crucial because truncated or incomplete sequences are often produced during synthesis. These truncated sequences must be removed to obtain high-purity oligonucleotides suitable for downstream applications like PCR, gene synthesis, or therapeutic use.
Applications of Phosphoramidite Synthesis
The phosphoramidite method is central to many areas of molecular biology, biotechnology, and medical research:
Primer and Probe Design: Oligonucleotides synthesized by this method are used as primers for PCR, qPCR, and other amplification techniques. They are also used as probes in hybridization assays such as Southern and Northern blotting.
Gene Synthesis: Short oligonucleotides can be synthesized and then assembled into longer sequences or entire genes using methods like PCR or enzymatic ligation.
Therapeutics: Synthetic oligonucleotides, such as antisense oligonucleotides, siRNAs, and aptamers, are used as therapeutic agents to regulate gene expression or bind to specific proteins or RNA molecules in cells.
Diagnostics: Oligonucleotides are used as molecular probes for detecting specific sequences of DNA or RNA in diagnostic assays, including next-generation sequencing (NGS), microarrays, and CRISPR-based diagnostics.
The phosphoramidite method represents a highly efficient, scalable, and reliable way to synthesize short DNA sequences. With the ability to automate this process and the high coupling efficiencies achieved, it remains the dominant technique for producing oligonucleotides for research, diagnostics, and therapeutics. Despite some challenges with length limitations and purification requirements, the phosphoramidite method is integral to modern molecular biology and synthetic biology, providing researchers with the precise DNA sequences they need for cutting-edge applications.


🧬 Support Independent Biotech Journalism 🧬
At BiotechnologyReviews.com, we’re committed to delivering in-depth, science-driven content that explores the cutting edge of genetics, molecular biology, and therapeutic innovation — all free and accessible to readers worldwide.
If you value high-quality, expertly researched articles on breakthroughs like epigenetic editing, gene therapy, and CRISPR-based technologies, we invite you to support our work.
Your pledge helps us:
Publish rigorous articles free from clickbait and hype
Cover underreported topics shaping the future of medicine and biotech
Keep our content independent, ad-light, and accessible to all
🔗 Make a difference — pledge your support today at https://lnkd.in/dDYUMY5g
Together, we can empower science-literate conversation and drive forward a more informed biotech future.