What Is Oligonucleotide Synthesis? Microarray-based oligonucleotide synthesis
Learn how scientists create custom DNA and RNA strands for use in research, diagnostics, and drug development.
This first article will cover method 2. Microarray-based oligonucleotide synthesis
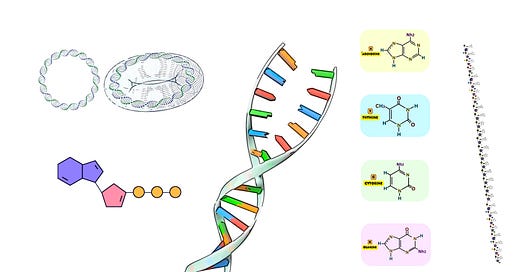
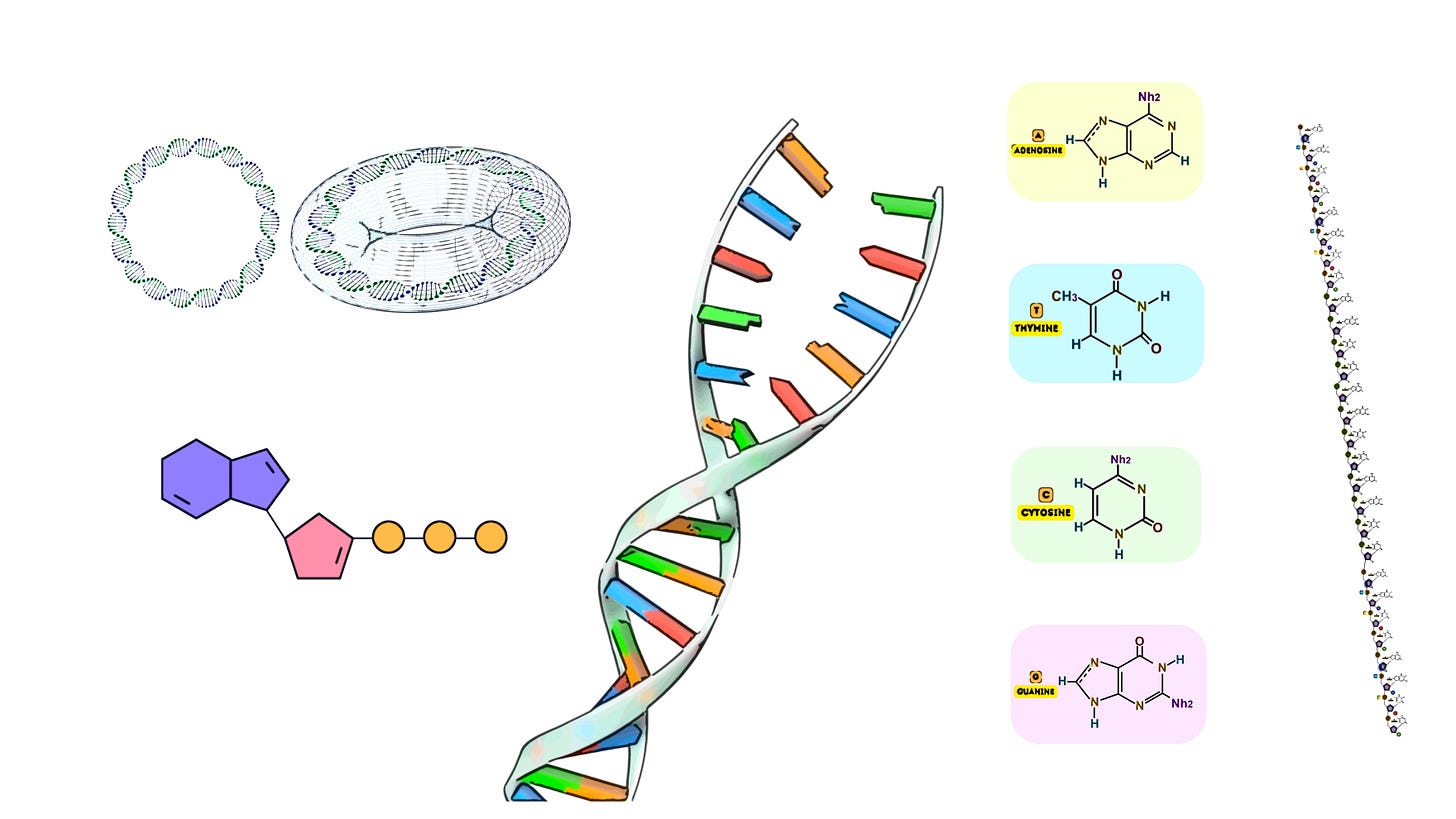
Oligonucleotide synthesis represents a foundational technique in molecular biology, biotechnology, and genomics, enabling the creation of custom-designed short sequences of nucleotides—DNA or RNA fragments—crucial for numerous applications such as genetic testing, diagnostics, gene editing, synthetic biology, and therapeutic development. The ability to synthesize these molecules with high specificity and precision has revolutionized the study of nucleic acids and facilitated a broad range of innovations across multiple scientific disciplines. Typically ranging between 5 and 100 nucleotides in length, oligonucleotides serve as primers for PCR, molecular probes, antisense therapies, and components in gene assembly, underscoring their indispensable role in both experimental and applied molecular sciences.
As this article series unfolds, we’ll explore each method in detail—covering workflows, reagents, advantages, limitations, and real-world applications across biotech, pharma, and academic research. This first article will cover method 2.
Microarray-based synthesis
Microarray-based synthesis is a cutting-edge method that enables the simultaneous synthesis of thousands to millions of different oligonucleotide sequences on a solid surface, typically a microchip. This approach has revolutionized high-throughput DNA synthesis, providing the ability to generate vast libraries of oligonucleotides in parallel. These sequences can then be used in applications ranging from genome-wide studies to synthetic biology, where large-scale, combinatorial experiments are essential.
In this process, DNA sequences are built up in a parallel and spatially defined manner, meaning that each oligonucleotide grows in a specific spot on the chip. The use of precise light or chemical control allows for the sequential addition of nucleotides, ensuring that each spot contains a unique sequence.
Let’s break down the microarray-based synthesis process in more technical detail.
Key Components of Microarray-Based Synthesis
Before discussing the process, it's important to understand the essential components that enable this technology:
Microarray Substrate: The synthesis takes place on a flat solid surface, usually made of glass or silicon. The surface is divided into thousands to millions of microscopic features, where each feature acts as an independent reaction site for growing unique oligonucleotides.
Photoprotected Phosphoramidite Chemistry: The synthesis of oligonucleotides on a microarray typically uses phosphoramidite chemistry, similar to the process used in traditional solid-phase oligonucleotide synthesis. However, the key innovation in microarray-based synthesis is the control over light-activated deprotection of the growing DNA chains.
Photolithography or Inkjet Printing: Microarray synthesis uses precise control over where nucleotide additions occur, either by using photolithography (light-directed synthesis) or inkjet printing technology to deliver chemical reagents selectively to different regions of the microarray.
Protecting Groups: Just as in the phosphoramidite method, each nucleotide added during the synthesis is protected by groups like DMT (dimethoxytrityl), which block reactive functional groups until they are needed for the next addition step. These protecting groups are removed in a highly controlled fashion to ensure correct sequence assembly.
Step-by-Step Process of Microarray-Based DNA Synthesis
There are two primary methods of performing microarray-based DNA synthesis: photolithography-based synthesis and inkjet printing-based synthesis. Both methods involve the sequential addition of nucleotide monomers to growing DNA chains on the surface of the microarray.
Photolithography-Based DNA Synthesis
Photolithography-based DNA synthesis is the most widely used method and operates similarly to the techniques used in semiconductor manufacturing. The critical feature of this method is the light-directed removal of protecting groups from specific regions of the microarray, allowing selective nucleotide addition.
Here’s how it works:
Surface Preparation: The microarray surface is first coated with a layer of nucleotides that are protected by a photolabile protecting group at the 5’-OH position. This group prevents uncontrolled coupling of nucleotides until it is removed by exposure to light.
Photomasking: A photomask is used to control which regions of the microarray are exposed to UV light. The photomask is essentially a stencil that allows light to reach only specific areas, leaving the rest of the surface untouched.
Deprotection via Light Exposure: In the regions where light passes through the photomask, the photolabile protecting groups on the nucleotides are removed. This exposes the 5’-hydroxyl groups on the growing oligonucleotides at those specific locations.
Nucleotide Addition (Coupling): The microarray is then flooded with a solution containing the next phosphoramidite nucleotide, which only couples to the deprotected regions of the surface. The coupling occurs via the standard phosphoramidite chemistry, forming a phosphite triester bond between the nucleotides.
Capping and Oxidation: As with traditional phosphoramidite synthesis, unreacted sites are capped to terminate any oligonucleotides that did not react during the coupling step, and the newly formed phosphite triester bond is oxidized to a more stable phosphate triester.
Repetition of Cycles: The process is repeated in cycles. A new photomask is applied, different regions are exposed to light, and another nucleotide is added. By controlling which areas of the microarray are exposed to light in each cycle, thousands to millions of unique oligonucleotides can be synthesized in parallel across the surface.
Final Cleavage and Purification: Once the synthesis is complete, the oligonucleotides are cleaved from the surface and collected. Depending on the application, further purification may be performed using HPLC or mass spectrometry.
Inkjet Printing-Based DNA Synthesis
Inkjet printing-based synthesis is an alternative approach that uses inkjet technology to deliver nucleotides directly to specific features on the microarray surface. This method avoids the use of light and photomasks, instead relying on the precise delivery of reagents.
Here’s how inkjet printing-based synthesis works:
Surface Preparation: The microarray surface is coated with solid-phase linker molecules, which anchor the growing oligonucleotides.
Nucleotide Delivery: An inkjet printing head is used to precisely dispense small droplets of the phosphoramidite nucleotide solutions onto specific features on the microarray. Each spot on the surface receives only the nucleotides that will extend the sequence at that location.
Selective Coupling: The nucleotide solution reacts with the exposed 5’-hydroxyl groups on the growing oligonucleotides at the targeted features. As in photolithography-based synthesis, the standard phosphoramidite chemistry forms the phosphite triester linkage between nucleotides.
Capping and Oxidation: After nucleotide addition, unreacted sites are capped to prevent undesired reactions, and oxidation stabilizes the newly formed linkage.
Repetition of Cycles: The inkjet printing head can be programmed to dispense different nucleotides to different locations on the microarray surface in each cycle. This allows for the simultaneous synthesis of many unique oligonucleotide sequences in parallel.
Final Cleavage and Purification: After all cycles of nucleotide addition are complete, the oligonucleotides are cleaved from the surface and purified, similar to the photolithography-based approach.
Comparison of Photolithography and Inkjet Printing Approaches
Photolithography Advantages:
Precision: The use of photomasks allows for highly precise control over which areas of the microarray surface are deprotected and thus where nucleotide addition occurs.
High density: Photolithography can achieve very high spatial resolution, allowing for extremely dense arrays of oligonucleotides to be synthesized. Modern systems can generate millions of unique sequences on a single chip.
Photolithography Limitations:
Mask complexity: The process requires the design and production of multiple photomasks, which can be complex and costly.
Limited flexibility: Once a photomask is designed, it is specific to a given oligonucleotide sequence. Changing sequences requires designing new masks, limiting the flexibility of this method for on-the-fly synthesis.
Inkjet Printing Advantages:
Flexibility: Since there are no photomasks involved, the system can easily switch between different sequences in real-time by simply changing the droplets dispensed by the inkjet head. This makes it more flexible for custom sequence synthesis.
Cost-effective: Inkjet printing does not require photomasks, which can reduce the overall cost of synthesis for small-scale or custom projects.
Inkjet Printing Limitations:
Lower resolution: Inkjet printing is generally less precise than photolithography, resulting in lower feature density on the microarray. This can limit the total number of unique oligonucleotides that can be synthesized on a single chip.
Reagent delivery: Droplet control and consistency can be challenging, especially at very small scales, and this can introduce variability in the synthesis process.
Key Applications of Microarray-Based Synthesis
Microarray-based oligonucleotide synthesis has been a game-changer in fields that require the high-throughput generation of large libraries of DNA sequences. Some of the major applications include:
Genomics and Gene Expression Studies: Microarrays are extensively used in genome-wide association studies (GWAS), where thousands of probes are synthesized to hybridize with specific DNA sequences in genomic samples. These probes allow researchers to study gene expression profiles, detect SNPs (single nucleotide polymorphisms), or identify genetic variations.
Synthetic Biology and Genetic Circuits: In synthetic biology, researchers need to assemble and test multiple genetic constructs. Microarray-based synthesis provides a fast and efficient way to generate large libraries of DNA sequences, which can be used to build complex genetic circuits or metabolic pathways.
CRISPR and Genome Editing: The microarray platform enables the parallel synthesis of large numbers of guide RNAs (gRNAs) for use in CRISPR-based genome editing. This allows for comprehensive screening experiments to identify the most effective gRNAs for targeting specific genomic loci.
Combinatorial Chemistry: Microarray-based synthesis is also used to generate vast libraries of oligonucleotides for combinatorial chemistry applications, including drug discovery, where researchers screen large numbers of potential drug candidates for specific binding or activity.
Mutagenesis and Protein Engineering: In studies that aim to explore protein function or engineer proteins with new properties, microarray-synthesized oligonucleotide libraries can be used to generate large numbers of mutant DNA sequences, which are then expressed and tested for functionality.
Advantages and Challenges of Microarray-Based Synthesis
Advantages:
Parallelism: The ability to synthesize millions of unique sequences simultaneously makes microarray-based synthesis incredibly powerful for large-scale applications, such as whole-genome studies or synthetic biology.
Scalability: Microarray technology is highly scalable, meaning that the cost per sequence decreases dramatically when large numbers of oligonucleotides are synthesized at once.
Customization: Researchers can design and synthesize customized oligonucleotide libraries for specific experimental needs, allowing for highly tailored experiments.
Challenges:
Sequence Length: The synthesis of longer oligonucleotides (above ~60 nucleotides) on microarrays can be challenging due to the cumulative errors introduced during each step of the synthesis process. As the sequence length increases, the overall fidelity of the oligonucleotides decreases.
Purity: The high-throughput nature of microarray-based synthesis means that the purity of the oligonucleotides may not be as high as in traditional, single-sequence synthesis. This can lead to mixed populations of sequences, requiring further purification or amplification steps.
Yield: The amount of oligonucleotide generated at each spot on the microarray is typically small (femtomoles to picomoles), which can limit downstream applications unless the sequences are amplified using methods like PCR.
Microarray-based oligonucleotide synthesis has transformed fields that require the simultaneous production of large numbers of DNA sequences. By miniaturizing and parallelizing the synthesis process, microarray technologies allow researchers to produce millions of oligonucleotides at once, making it a highly efficient and cost-effective platform for genomics, synthetic biology, and drug discovery. While challenges like sequence length limitations and purity remain, the rapid advances in this technology continue to expand its capabilities and applications.