What Is Oligonucleotide Synthesis? Terminal Deoxynucleotidyl Transferase (TdT) synthesis
Learn how scientists create custom DNA and RNA strands for use in research, diagnostics, and drug development.
This first article will cover method 5. Terminal Deoxynucleotidyl Transferase (TdT) synthesis
Oligonucleotide synthesis represents a foundational technique in molecular biology, biotechnology, and genomics, enabling the creation of custom-designed short sequences of nucleotides—DNA or RNA fragments—crucial for numerous applications such as genetic testing, diagnostics, gene editing, synthetic biology, and therapeutic development. The ability to synthesize these molecules with high specificity and precision has revolutionized the study of nucleic acids and facilitated a broad range of innovations across multiple scientific disciplines. Typically ranging between 5 and 100 nucleotides in length, oligonucleotides serve as primers for PCR, molecular probes, antisense therapies, and components in gene assembly, underscoring their indispensable role in both experimental and applied molecular sciences.
As this article series unfolds, we’ll explore each method in detail—covering workflows, reagents, advantages, limitations, and real-world applications across biotech, pharma, and academic research. This first article will cover method 5.
Terminal Deoxynucleotidyl Transferase (TdT) synthesis
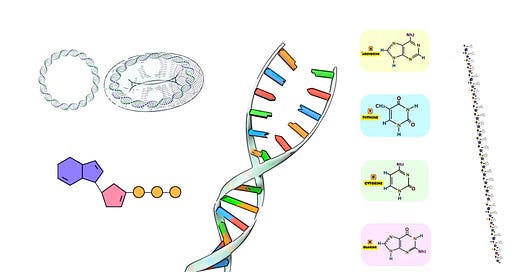
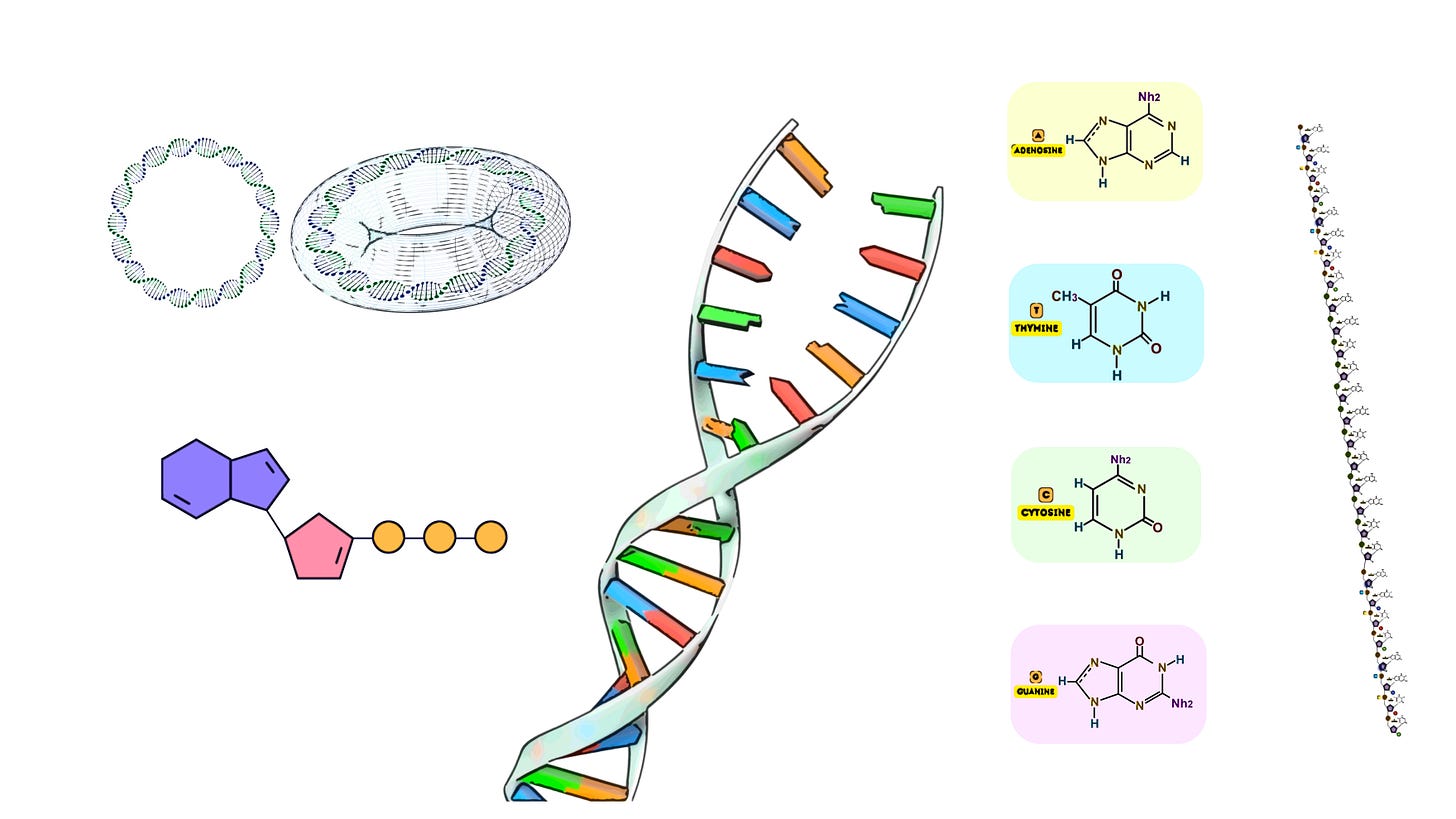
Terminal Deoxynucleotidyl Transferase (TdT) is a unique DNA polymerase that plays a critical role in both natural and synthetic DNA processes. Unlike traditional DNA polymerases, TdT is a template-independent polymerase, meaning it does not require a complementary DNA strand to guide the incorporation of nucleotides. Instead, it adds nucleotides to the 3’-OH end of a DNA molecule in a random or controlled manner, depending on the available nucleotides. This enzyme is used extensively in both immunology—where it is essential for generating antibody diversity—and molecular biology applications, such as introducing random or specific sequences to the ends of DNA molecules.
In this technical breakdown, we’ll dive deep into the mechanism, applications, and challenges associated with TdT-based synthesis, explaining how this enzyme is used and its significance in various fields.
Overview of Terminal Deoxynucleotidyl Transferase (TdT)
TdT is a DNA polymerase that adds deoxyribonucleotides to the 3’-OH terminus of single-stranded or double-stranded DNA without requiring a template strand. It belongs to the X family of DNA polymerases and is predominantly found in the vertebrate immune system, specifically in immature lymphocytes.
TdT is naturally involved in the process of V(D)J recombination, a key mechanism used by the immune system to generate the vast diversity of antibodies and T-cell receptors (TCRs). During V(D)J recombination, TdT randomly adds nucleotides to the ends of DNA segments being recombined, thereby increasing the diversity of the antigen receptor repertoire.
In molecular biology and synthetic biology, TdT’s template-independent activity can be harnessed for DNA labeling, randomized sequence generation, and synthetic oligonucleotide modification.
Key Components of TdT-Based DNA Synthesis
To understand TdT-based DNA synthesis, we must first identify the key components involved in the process:
Template DNA: While TdT does not require a template for nucleotide addition, it does need a 3’-OH group on the DNA. This could be a single-stranded DNA (ssDNA) or the 3’ overhang of a double-stranded DNA (dsDNA) molecule. TdT preferentially adds nucleotides to single-stranded 3' ends.
dNTPs (Deoxynucleotide Triphosphates): TdT adds deoxynucleotide triphosphates (dNTPs) to the 3'-OH terminus of the DNA strand. These can be any of the four canonical nucleotides (dATP, dTTP, dGTP, dCTP), as well as modified nucleotides used for specific applications.
Cofactors: TdT requires divalent metal ions (typically Mg²⁺ or Co²⁺) to catalyze the nucleotide addition reaction. Co²⁺ tends to favor more random and efficient nucleotide addition, while Mg²⁺ can provide more control over the addition process.
Buffer Conditions: Optimal activity of TdT requires specific buffer conditions, typically involving a pH of around 7.0-7.5 and ionic strength provided by salts such as potassium chloride (KCl). The composition of the buffer can influence the enzyme's efficiency and the length of the nucleotide tail added.
Mechanism of TdT-Mediated DNA Synthesis
The mechanism of TdT-based DNA synthesis can be broken down into the following steps:
Binding of the DNA Substrate
TdT recognizes and binds to a free 3'-OH group on the end of a DNA molecule. This can be a single-stranded DNA or a 3' overhang of a double-stranded DNA. The enzyme does not require a complementary template, but the substrate DNA must present a 3’-OH terminus, which serves as the starting point for nucleotide addition.
Nucleotide Binding
Once TdT is bound to the DNA, the nucleotide triphosphates (dNTPs) in the reaction are positioned by the enzyme. TdT uses its active site to interact with the incoming nucleotide’s triphosphate group and catalyzes the formation of a phosphodiester bond between the 3’-OH group of the terminal nucleotide and the incoming dNTP.
Nucleotide Addition
The enzyme facilitates the nucleophilic attack of the 3’-OH group on the alpha-phosphate of the dNTP, forming a new phosphodiester bond and releasing pyrophosphate (PPi) as a byproduct. The chain is elongated by one nucleotide, and the new terminal nucleotide now presents a 3’-OH group for further elongation.
Processive Addition
TdT can continue to add multiple nucleotides in a processive manner, meaning that it does not dissociate from the DNA after adding one nucleotide. Depending on the conditions and nucleotide availability, TdT can add anywhere from a few nucleotides to long homopolymeric tails of several hundred nucleotides in length.
When a mixture of nucleotides is present, TdT will add these nucleotides randomly to the 3' end of the DNA strand, unless special conditions or inhibitors are used to regulate nucleotide addition.
When modified nucleotides are used, TdT can be directed to add specific labels or functional groups to the 3’ end of the DNA, which can be used in applications such as labeling or bioconjugation.
Factors Controlling TdT Activity
While TdT is known for adding nucleotides randomly, its activity can be controlled by adjusting various factors in the reaction:
Cofactors (Mg²⁺ vs. Co²⁺):
Mg²⁺ is the preferred cofactor in most TdT reactions, as it ensures relatively controlled and slower nucleotide addition. It allows for better incorporation of specific nucleotides and limits excessive tailing.
Co²⁺ is a stronger activator of TdT, promoting more processive and random nucleotide addition. Co²⁺ leads to the generation of longer nucleotide tails, which can be useful in applications where long homopolymeric stretches or extensive tailing are desired.
Nucleotide Concentration:
High concentrations of dNTPs lead to more rapid and extensive tailing, whereas low concentrations allow for slower and more controlled nucleotide addition.
By providing only one type of nucleotide (e.g., only dATP), TdT can be used to add homopolymeric tails of a single nucleotide to the 3’ end of the DNA.
Modified Nucleotides:
Modified nucleotides, such as those with fluorescent labels, biotinylation, or blocking groups, can be used with TdT to specifically tag or label DNA molecules.
TdT can incorporate modified nucleotides at the 3’ end, providing a simple method for adding functional groups to DNA, which is useful in probe design, DNA sequencing, and molecular diagnostics.
Buffer Composition:
The pH and ionic strength of the buffer can influence TdT’s activity. Optimal activity typically occurs at pH 7.5 with KCl or NaCl providing the appropriate ionic strength.
Some buffer compositions favor more controlled and shorter additions, while others enhance random addition of longer tails.
Applications of TdT Synthesis
TdT has several specialized applications in both immunology and molecular biology, taking advantage of its template-independent nature and the ability to add nucleotides randomly or in a controlled fashion.
Antibody Diversity and V(D)J Recombination
TdT plays a central role in the immune system by contributing to the generation of diverse antigen receptor repertoires. During the development of B cells and T cells, the genes encoding for the variable (V), diversity (D), and joining (J) segments of antibodies and T-cell receptors are rearranged through a process called V(D)J recombination.
TdT adds random nucleotides to the ends of the recombining DNA segments, increasing diversity by generating N-region insertions. This results in a highly variable sequence at the junctions between the V, D, and J segments, which allows the immune system to recognize a vast array of antigens.
The random addition of nucleotides by TdT is critical for the creation of diverse antibody paratopes (the part of the antibody that binds to an antigen) and T-cell receptor epitopes.
DNA End Labeling
One of the most common applications of TdT in molecular biology is DNA end labeling. TdT can be used to add nucleotides that are labeled with various chemical groups or fluorescent tags to the 3’ end of a DNA molecule. This is especially useful in applications such as:
Fluorescent labeling: Incorporation of fluorescently labeled nucleotides (e.g., fluorescein-dUTP) at the 3' ends of DNA enables the visualization of specific DNA molecules during microscopy or flow cytometry.
Biotin labeling: The addition of biotinylated nucleotides allows for the subsequent detection of DNA using streptavidin-conjugated probes, which can be used in blotting, pull-down assays, or diagnostic assays.
Radioactive labeling: TdT can also incorporate nucleotides labeled with radioactive isotopes like 32P for use in autoradiography or other traditional detection methods.
3’ End Tailing for Cloning and Ligation
In cloning experiments, TdT is used to add homopolymeric tails to the 3' ends of DNA molecules. This technique can facilitate the ligation of DNA fragments or the cloning of PCR-amplified products into vectors.
Homopolymer Tailing: TdT can add a poly(A) or poly(T) tail to the 3’ end of a DNA fragment. These homopolymeric tails can then hybridize with complementary oligonucleotide tails (e.g., poly(T) to poly(A)) to facilitate cloning without the need for restriction enzymes.
T-overhang cloning: TdT is often used in the preparation of T-overhangs on linearized vectors for TA cloning, a method where PCR products with A-overhangs (from Taq polymerase) can be efficiently ligated into the vector.
Random Sequence Generation for Directed Evolution
TdT’s ability to add nucleotides randomly to the 3' end of DNA can be used to generate randomized DNA sequences, which are critical for applications such as directed evolution, where libraries of variants are created and screened for desired properties (e.g., enzyme activity or ligand binding affinity).
By controlling the types of nucleotides present in the reaction, researchers can create randomized oligonucleotide libraries with specific nucleotide compositions, which are then subjected to evolutionary pressure through techniques like phage display or ribosome display.
TUNEL Assay for Apoptosis Detection
TdT is the enzyme used in the TUNEL assay (Terminal deoxynucleotidyl transferase dUTP nick end labeling), a method to detect DNA fragmentation that results from apoptotic cell death.
During apoptosis, DNA is fragmented into small pieces. In the TUNEL assay, TdT adds labeled dUTP molecules to the 3’-OH ends of DNA breaks, allowing for the identification and quantification of apoptotic cells via microscopy or flow cytometry.
Advantages of TdT Synthesis
Template Independence: TdT is unique among DNA polymerases because it does not require a complementary template strand, allowing it to be used in applications that require random or template-independent DNA extension.
Versatility: TdT can incorporate both natural and modified nucleotides, enabling the addition of labeled, fluorescent, or chemically modified nucleotides for downstream detection or molecular engineering.
Customization: TdT’s activity can be controlled to add random sequences or specific labels, making it highly flexible for a range of molecular biology and synthetic biology applications.
Challenges and Limitations of TdT Synthesis
Despite its many applications, TdT-based synthesis presents several challenges:
Lack of Sequence Control: While TdT can add nucleotides randomly or homopolymerically, controlling the precise sequence of nucleotides added is difficult without using modified nucleotides or specialized reaction conditions. This limits its use in applications that require sequence precision.
Limited Processivity: TdT’s processivity can vary depending on the cofactor and conditions, which may result in shorter or longer than desired nucleotide additions, making fine-tuned control of the tail length challenging.
Substrate Limitations: TdT prefers single-stranded 3' overhangs and may exhibit reduced activity or efficiency when adding nucleotides to blunt-ended or double-stranded DNA, unless appropriate modifications are made to the reaction conditions.
Terminal Deoxynucleotidyl Transferase (TdT) is a versatile and powerful tool in both immunology and molecular biology, enabling the template-independent addition of nucleotides to the 3' end of DNA molecules. From generating antibody diversity in the immune system to labeling DNA for molecular diagnostics and generating random sequence libraries, TdT plays an essential role in a wide range of applications. While its lack of sequence specificity limits some applications, ongoing research and innovations in enzyme engineering continue to expand its utility in the lab.
Specialist Enzymes for synthesis
In oligonucleotide manufacturing, a range of specialist enzymes are employed to facilitate stepwise addition of nucleotides or to modify nucleic acids. These enzymes are particularly useful for enzymatic synthesis approaches, where precision and sequence control are critical. Below is a list of enzymes, besides Terminal Deoxynucleotidyl Transferase (TdT), that are used for stepwise nucleotide addition or nucleic acid modification:
DNA Polymerase I (Klenow Fragment)
Function: The Klenow fragment is a large fragment of DNA Polymerase I from E. coli that retains the polymerase activity but lacks the 5' to 3' exonuclease activity. It is commonly used for stepwise addition of nucleotides to single-stranded DNA and for fill-in reactions on recessed 3' ends of DNA.
Application: Frequently used for generating blunt ends by filling in sticky ends of double-stranded DNA and for synthesizing complementary strands during DNA replication experiments.
T7 DNA Polymerase
Function: A highly processive DNA polymerase that is often used in DNA synthesis and sequencing applications. T7 DNA polymerase has high fidelity and is involved in synthesis of DNA strands by adding nucleotides complementary to a template strand.
Application: Used in sequencing reactions and in applications where high-fidelity replication is required.
Taq DNA Polymerase
Function: A thermostable polymerase from Thermus aquaticus, Taq DNA polymerase is typically used in PCR for amplifying DNA by adding nucleotides to the growing DNA chain during thermal cycling. It lacks proofreading activity, but its ability to function at high temperatures makes it ideal for PCR.
Application: PCR amplification, DNA sequencing, and oligonucleotide synthesis where thermostable activity is required.
Pfu DNA Polymerase
Function: An enzyme from Pyrococcus furiosus with high-fidelity DNA polymerization due to its 3’ to 5’ exonuclease (proofreading) activity. Pfu is more accurate than Taq polymerase and is used for stepwise synthesis of high-fidelity DNA fragments.
Application: PCR, gene synthesis, and applications requiring high accuracy in the synthesis of DNA strands.
T4 DNA Ligase
Function: While not a polymerase, T4 DNA ligase is crucial for nucleotide assembly during oligonucleotide synthesis, as it catalyzes the formation of phosphodiester bonds between adjacent 5’-phosphate and 3’-hydroxyl groups of nucleotides. It is used to ligate fragments of DNA into a continuous strand.
Application: Gene synthesis, cloning, and constructing longer DNA sequences from smaller oligonucleotides.
T4 RNA Ligase
Function: Similar to T4 DNA ligase, but specific for RNA ligation. It catalyzes the formation of a phosphodiester bond between the 3’-OH and 5’-phosphate ends of RNA strands. This enzyme is useful in RNA sequencing and modification.
Application: Ligation of RNA oligonucleotides, RNA sequencing, and RNA labeling.
Polynucleotide Kinase (T4 PNK)
Function: T4 Polynucleotide Kinase adds a phosphate group to the 5'-hydroxyl terminus of nucleotides, allowing subsequent ligation by T4 DNA ligase. It is not directly involved in stepwise nucleotide addition but plays a crucial role in preparing oligonucleotides for ligation.
Application: Preparation of oligonucleotides for cloning, radiolabeling, and phosphorylation of DNA/RNA ends for ligation.
T7 RNA Polymerase
Function: T7 RNA polymerase synthesizes RNA in a stepwise manner by transcribing DNA templates that contain a specific T7 promoter sequence. It is used for in vitro transcription of RNA.
Application: Synthesis of RNA for structural studies, functional assays, and synthetic biology applications involving RNA.
DNA Primase
Function: DNA primase synthesizes RNA primers during DNA replication. These primers are required for initiating the stepwise addition of nucleotides by DNA polymerases during replication.
Application: DNA replication studies, oligo-primed synthesis, and in vitro DNA synthesis reactions where primers are required.
Poly(A) Polymerase
Function: Adds poly(A) tails to the 3' end of mRNA molecules. This enzyme is crucial for RNA maturation and stability, and it is also used in RNA tailing experiments in molecular biology.
Application: mRNA polyadenylation, labeling RNA molecules, and synthetic biology applications involving RNA.
Exonuclease III
Function: Exonuclease III catalyzes the removal of nucleotides from the 3’ ends of DNA molecules in a stepwise manner, creating blunt ends. It also has exonuclease activity but can be used to generate stepwise reductions in oligonucleotide length for various applications.
Application: Deletion mutagenesis, creating blunt ends for cloning, and controlled oligonucleotide digestion.
Phi29 DNA Polymerase
Function: A highly processive polymerase with strand-displacement activity, allowing for continuous synthesis of long DNA strands. It can add nucleotides in a stepwise manner without the need for a template reset.
Application: Rolling circle amplification (RCA), whole-genome amplification, and constructing long DNA sequences.
Reverse Transcriptase (RT)
Function: Reverse transcriptase catalyzes the synthesis of complementary DNA (cDNA) from an RNA template in a stepwise manner. It is critical for converting RNA into DNA in applications such as cDNA library construction and gene expression studies.
Application: Synthesis of cDNA from mRNA, reverse transcription PCR (RT-PCR), and cloning of RNA sequences.
Summary, Enzymes for Stepwise Nucleotide Addition
These enzymes, often in combination, allow for controlled and precise addition of nucleotides in various oligonucleotide synthesis and nucleic acid modification processes. Depending on the type of nucleic acid (DNA or RNA), and the specificity required (e.g., for cloning, labeling, or gene synthesis), different enzymes can be utilized for stepwise addition of bases. Each enzyme is optimized for different steps of oligo manufacturing, offering a high degree of control over sequence fidelity, length, and function.
Example case: Telomerase
Telomerase is a specialized reverse transcriptase enzyme that extends the ends of linear chromosomes by adding repetitive DNA sequences to the telomeres, which are the protective caps at the ends of eukaryotic chromosomes. Telomerase plays a critical role in maintaining chromosome stability and is especially active in stem cells, germ cells, and certain cancer cells.
How Telomerase Works
Telomerase is unique in that it contains its own RNA template within the enzyme complex, which it uses to guide the stepwise addition of nucleotides to the 3' end of the DNA strand. This makes it a template-dependent DNA polymerase with a built-in RNA guide, distinguishing it from the template-independent polymerases like Terminal Deoxynucleotidyl Transferase (TdT). Here’s how it works in more detail:
RNA Template: Telomerase contains an integral RNA molecule, part of the telomerase ribonucleoprotein complex, which serves as the template for synthesizing the telomeric repeat sequences. In humans, the RNA template within telomerase directs the addition of the sequence TTAGGG.
Binding to the DNA Substrate: Telomerase binds to the 3' overhang of the telomere at the end of the chromosome. This region is single-stranded due to the natural degradation of the lagging strand during replication, making it an ideal substrate for the enzyme.
Stepwise Addition of Nucleotides: Using its RNA template, telomerase catalyzes the stepwise addition of nucleotides to the 3’ end of the chromosome. For example, in human cells, telomerase adds the repeating sequence TTAGGG to the ends of the telomeres.
Dissociation and Resetting: Once a telomeric repeat is added, telomerase can dissociate and then realign itself on the newly extended 3' end to add another repeat. This process can be repeated multiple times, leading to the extension of the telomere by many repeated sequences.