What Is Oligonucleotide Synthesis? Gene Synthesis via Assembly of Overlapping Oligonucleotides
Learn how scientists create custom DNA and RNA strands for use in research, diagnostics, and drug development.
This first article will cover method 6. Gene Synthesis via Assembly of Overlapping Oligonucleotides
Oligonucleotide synthesis represents a foundational technique in molecular biology, biotechnology, and genomics, enabling the creation of custom-designed short sequences of nucleotides—DNA or RNA fragments—crucial for numerous applications such as genetic testing, diagnostics, gene editing, synthetic biology, and therapeutic development. The ability to synthesize these molecules with high specificity and precision has revolutionized the study of nucleic acids and facilitated a broad range of innovations across multiple scientific disciplines. Typically ranging between 5 and 100 nucleotides in length, oligonucleotides serve as primers for PCR, molecular probes, antisense therapies, and components in gene assembly, underscoring their indispensable role in both experimental and applied molecular sciences.
As this article series unfolds, we’ll explore each method in detail—covering workflows, reagents, advantages, limitations, and real-world applications across biotech, pharma, and academic research. This first article will cover method 6.
Gene Synthesis via Assembly of Overlapping Oligonucleotides
Gene synthesis via assembly of overlapping oligonucleotides is a powerful molecular biology technique that allows for the de novo construction of entire genes or even larger DNA constructs from short synthetic oligonucleotides. This method is commonly used in synthetic biology, protein engineering, and genomics for creating custom-designed genetic sequences without the need for a template. Instead of cloning from a biological source, gene synthesis enables the design and construction of completely novel DNA sequences with high precision. The technique involves the design of short, overlapping oligonucleotides that are complementary to each other at their termini, which allows for precise assembly through hybridization followed by enzymatic assembly using methods like PCR or ligase-mediated assembly.
This article will explore the details of how gene synthesis via overlapping oligonucleotides is achieved, including the principles behind oligonucleotide design, enzymatic assembly techniques, and the challenges and advantages of this method. We will also cover its applications and how it compares to other gene synthesis approaches.
Key Concepts in Gene Synthesis via Oligonucleotide Assembly
Gene synthesis via assembly of overlapping oligonucleotides can be broken down into several key steps. These include the design of overlapping oligonucleotides, their annealing (or hybridization) to form a continuous DNA sequence, and the subsequent ligation or PCR amplification to create a full-length gene.
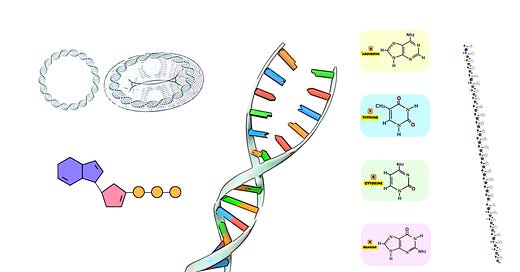
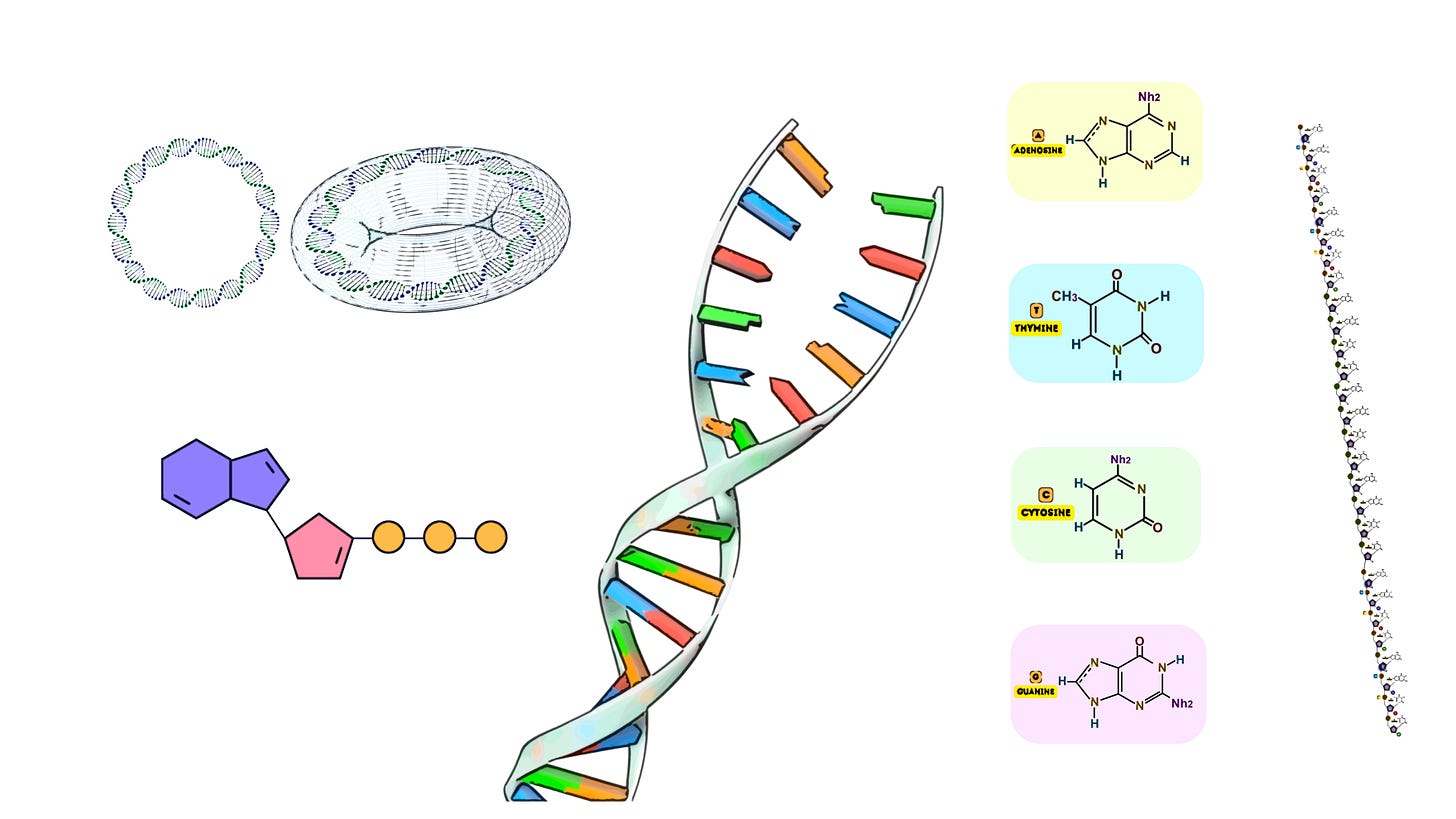
Overlapping Oligonucleotides
Oligonucleotides, or oligos, are short strands of single-stranded DNA, typically ranging from 20 to 60 nucleotides in length. In gene synthesis, these oligos are designed with overlapping regions—typically 15 to 20 nucleotides—so that adjacent oligos can hybridize, or anneal, to each other based on their complementary sequences.
The overlapping regions serve two important purposes:
Hybridization: The overlaps ensure that each oligonucleotide can find its complement, allowing for accurate and specific annealing into a continuous strand.
Assembly: These overlaps provide a scaffold for enzymatic assembly processes, such as PCR or ligation, that join the oligos into a longer contiguous sequence.
Assembly Strategy
There are two main enzymatic approaches for assembling overlapping oligonucleotides into full-length DNA constructs:
PCR-Based Assembly: PCR is used to amplify the overlapping oligonucleotides, filling in the gaps between oligos and assembling them into a full-length double-stranded DNA.
Ligation-Based Assembly: DNA ligases are used to join the adjacent oligonucleotides at their overlapping regions to create a continuous DNA sequence.
Each approach has its own strengths and is chosen based on the specific requirements of the synthesis project, such as the length of the gene or sequence, fidelity, and the desired output.
Step-by-Step Process of Gene Synthesis via Overlapping Oligonucleotides
Let’s explore the entire process, from the design phase to the assembly of the full gene.
Oligonucleotide Design
The first and most critical step in gene synthesis via oligonucleotide assembly is the design of oligos. This is typically done with the help of bioinformatics tools that break down a target gene or desired DNA sequence into smaller, overlapping oligos. Key considerations during the design phase include:
Length of Oligos: Oligos are typically designed to be between 20 and 60 nucleotides long. Longer oligos tend to have higher chances of synthesis errors, while shorter oligos require more steps for assembly, increasing the complexity of the synthesis process.
Overlap Region: The overlapping regions between oligos are usually designed to be 15-20 nucleotides long. This provides sufficient base pairing for stable hybridization without excessive redundancy, which could increase the likelihood of errors during synthesis.
GC Content: The oligonucleotides should have a balanced GC content (40-60%) to ensure efficient hybridization during annealing. Very high GC content can lead to the formation of secondary structures, while very low GC content can result in weak hybridization.
Codon Optimization: When designing a gene for expression in a specific organism, the codons may be optimized to reflect the preferred codon usage of the target host organism. Codon optimization helps improve translation efficiency and protein expression levels in the host.
Once the oligonucleotides are designed, they are synthesized using phosphoramidite chemistry or purchased from commercial suppliers.
Annealing (Hybridization)
After the oligonucleotides are synthesized, they are mixed together in equimolar amounts and subjected to annealing conditions that allow the overlapping regions to form complementary base pairs. The temperature is gradually lowered from a denaturation temperature (around 95°C) to an annealing temperature (around 55-65°C), enabling the oligos to find their complementary partners and form a continuous, double-stranded DNA molecule.
The success of this step is highly dependent on the quality of the oligos and the stringency of the annealing conditions. Mismatches or errors in synthesis can result in incomplete or incorrect assembly, which may require optimization of the oligo design or annealing parameters.
Enzymatic Assembly: PCR-Based Method
Once the oligonucleotides have annealed, PCR (Polymerase Chain Reaction) can be used to extend and assemble the overlapping fragments into a full-length gene. The key steps in PCR-based gene synthesis include:
Initial Annealing: The short oligos anneal to one another based on their overlapping regions.
Primer Extension: DNA polymerase (e.g., Taq polymerase or Pfu polymerase) fills in the gaps between the overlapping oligos, extending from the 3' end of one oligo to the 5' end of the adjacent oligo.
Amplification: In subsequent cycles of PCR, the newly synthesized DNA serves as a template for further amplification. This results in the exponential amplification of the full-length gene.
In PCR-based assembly, the outermost oligonucleotides serve as primers for the amplification process. These outer primers are complementary to the ends of the desired gene and help amplify the entire construct. The process typically involves 25-35 cycles of PCR, with denaturation at 95°C, annealing at 55-65°C, and extension at 72°C.
This method is highly efficient for synthesizing genes that are several hundred base pairs long and has the advantage of high yield due to PCR amplification. However, the presence of errors in the initial oligonucleotides can be propagated during amplification, so high-fidelity polymerases are often used to minimize errors.
Enzymatic Assembly: Ligation-Based Method
For larger or more complex assemblies, an alternative to PCR is ligation-based gene synthesis. This method uses DNA ligases to join adjacent oligonucleotides at their overlapping regions without the need for amplification.
The process involves the following steps:
Annealing: As in the PCR method, the oligos are designed with overlapping regions and anneal to form complementary base pairs.
Ligation: A DNA ligase (typically T4 DNA ligase) is added to catalyze the formation of phosphodiester bonds between the 5'-phosphate and 3'-OH groups at the junctions between oligonucleotides.
Final Assembly: After ligation, the full-length gene can be cloned into a plasmid for further amplification and sequencing.
Ligation-based assembly is advantageous when synthesizing longer DNA sequences or assembling multiple fragments, as it does not rely on the exponential amplification of PCR, which can sometimes introduce errors. This method is particularly useful for assembling genes or constructs that are over 1-2 kilobases (kb) in length or for assembling entire synthetic genomes.
Cloning and Verification
Once the oligonucleotides have been successfully assembled into a full-length gene, the next step is to clone the product into a suitable vector for further propagation and manipulation. The typical process involves:
Cloning into a Plasmid Vector: The synthesized gene is inserted into a plasmid vector that contains a selectable marker (e.g., antibiotic resistance) and regulatory elements for gene expression. The ligated product or PCR product is treated with restriction enzymes and cloned into the vector using T4 DNA ligase.
Transformation and Amplification: The recombinant plasmid is then introduced into a bacterial host (e.g., E. coli) via transformation. The bacterial cells amplify the plasmid, allowing for large-scale production of the synthesized gene.
Sequence Verification: After amplification, the synthesized gene is verified using Sanger sequencing or next-generation sequencing (NGS) to ensure that the desired sequence was correctly assembled. This step is critical because errors in oligonucleotide synthesis or assembly can lead to incorrect sequences, which could affect downstream applications.
Challenges in Gene Synthesis via Overlapping Oligonucleotides
Although gene synthesis via assembly of overlapping oligonucleotides is a powerful tool, there are several challenges associated with this technique:
Synthesis Errors in Oligonucleotides:
The phosphoramidite synthesis method used to create oligonucleotides is prone to errors, particularly when synthesizing longer oligos (>60 nucleotides). Even a small error rate can result in incorrect assembly, which can be problematic for larger genes.
Error rates can accumulate when assembling a large number of oligos, making it essential to use high-quality synthesis and purification methods.
Secondary Structures:
The presence of secondary structures, such as hairpins or G-quadruplexes, in oligonucleotides can interfere with proper annealing and assembly. These structures may prevent correct base pairing between oligos and reduce the efficiency of the assembly process.
Careful attention to the GC content and sequence design is required to minimize secondary structure formation.
Assembly of Long Sequences:
As the length of the desired gene increases, the complexity of assembly also increases. Synthesizing genes longer than 1-2 kb may require multiple rounds of assembly, using PCR or ligation to join smaller fragments into larger constructs.
Error Propagation:
Errors introduced during the initial oligonucleotide synthesis or PCR amplification can be propagated during the assembly process. High-fidelity polymerases, such as Pfu or Q5, are often used to minimize errors during PCR-based assembly.
Cost and Time:
The cost of oligonucleotide synthesis and assembly can be significant, especially for longer or more complex genes. Although prices have decreased in recent years, large-scale gene synthesis projects can still be costly and time-consuming.
Advantages of Gene Synthesis via Overlapping Oligonucleotides
Despite the challenges, gene synthesis via assembly of overlapping oligonucleotides offers several key advantages:
Flexibility:
This method allows for the design and synthesis of completely novel DNA sequences, including synthetic genes, promoter sequences, and regulatory elements that do not exist in nature.
Codons can be optimized for specific expression systems, and non-standard amino acids or other modifications can be incorporated into the gene.
Precision:
By designing the oligonucleotides, researchers can create tailor-made genes with specific features, including mutation insertion, domain swapping, and sequence optimization.
Scalability:
This approach is scalable from short DNA sequences (several hundred base pairs) to entire synthetic genomes, enabling large-scale applications in synthetic biology and genetic engineering.
No Template Required:
Gene synthesis via oligonucleotide assembly does not require a template DNA from a biological source, which is particularly useful for synthesizing genes that are difficult to isolate from natural organisms.
Applications of Gene Synthesis via Overlapping Oligonucleotides
The ability to synthesize custom genes from scratch has revolutionized multiple fields of biology and biotechnology. Some key applications include:
Synthetic Biology:
Gene synthesis is fundamental to synthetic biology, where researchers design and build novel biological systems, such as engineered metabolic pathways, gene circuits, and synthetic organisms.
Protein Engineering:
By synthesizing genes that encode for engineered proteins, researchers can design proteins with improved stability, altered binding properties, or new enzymatic activities. This has applications in biopharmaceuticals, enzyme design, and structural biology.
Vaccine Development:
Gene synthesis is used to produce genes encoding antigens for vaccines, allowing researchers to rapidly develop and test new vaccines for emerging diseases.
Functional Genomics:
Custom-designed genes can be synthesized and introduced into cells or organisms to study the function of genetic elements or to create reporter constructs for monitoring gene expression.
CRISPR/Cas9 Technology:
Gene synthesis is used to create guide RNA (gRNA) sequences for CRISPR/Cas9 genome editing, enabling precise targeting of specific genetic loci.
Gene synthesis via assembly of overlapping oligonucleotides is a highly versatile and powerful tool in molecular biology and synthetic biology. It allows for the construction of de novo genes and other DNA constructs by assembling short, overlapping oligonucleotides into full-length sequences using either PCR-based or ligation-based methods. Despite challenges like oligonucleotide synthesis errors and secondary structure formation, this method provides unmatched flexibility and precision in gene design and synthesis. As technologies continue to improve, gene synthesis will remain an essential technique for genetic engineering, synthetic biology, and biotechnology applications.
Conclusion
Oligonucleotide synthesis stands as a fundamental pillar in the landscape of molecular biology, genetics, and biotechnology. The capacity to chemically and enzymatically construct short sequences of DNA and RNA has not only advanced our understanding of biological systems but also revolutionized the way we manipulate genetic material for a vast array of applications. These applications span fundamental research, clinical diagnostics, therapeutics, synthetic biology, and genomic engineering. The continual refinement and diversification of oligonucleotide synthesis technologies have dramatically increased the precision, scalability, and efficiency with which these sequences can be generated, fostering new possibilities in both research and industry.
The phosphoramidite method, which remains the most widely adopted and robust approach for synthesizing high-purity oligonucleotides, has facilitated the rapid and efficient production of custom DNA and RNA sequences. Its solid-phase synthesis mechanism, which follows a tightly regulated cycle of detritylation, coupling, capping, and oxidation, enables the stepwise construction of oligonucleotides with high fidelity and reproducibility. Despite its limitations in producing longer sequences due to cumulative inefficiencies in the coupling reactions, phosphoramidite synthesis has achieved remarkable scalability and automation, supporting industrial-scale production of oligonucleotides for use in PCR primers, antisense oligonucleotides, probes, and other molecular tools. The development of automated DNA synthesizers has further improved the precision of this technique, allowing laboratories to produce oligonucleotides with minimal human intervention, thus reducing error rates and increasing throughput.
In parallel, microarray-based synthesis has transformed the field of genomics and synthetic biology by enabling the production of vast libraries of oligonucleotide sequences in parallel on a single substrate. This high-throughput technique, which employs either photolithographic methods or inkjet printing technologies, allows for the simultaneous synthesis of thousands to millions of unique sequences, making it indispensable for large-scale studies such as SNP genotyping, CRISPR screening, and gene expression profiling. However, the limitation on sequence length due to lower coupling efficiencies in high-throughput settings remains a challenge. Nevertheless, this method has significantly accelerated research in genomics and synthetic biology, where the rapid synthesis of diverse DNA libraries is essential.
The polymerase chain reaction (PCR), while primarily recognized as an amplification technique, plays a crucial role in oligonucleotide synthesis and modification. PCR-based approaches such as overlap extension PCR and polymerase cycling assembly (PCA) allow for the synthesis of novel or modified DNA sequences by combining multiple oligonucleotides into larger, functional constructs. These methods have proven indispensable in cloning, mutagenesis, and gene assembly, where the need to create precise genetic modifications or entirely new gene sequences is paramount. PCR-based oligonucleotide synthesis provides the flexibility to generate highly specific sequences from minimal starting material, enabling rapid amplification and modification of target sequences, which is critical in fields ranging from synthetic biology to therapeutic gene editing.
The emergence of enzymatic DNA synthesis marks a promising shift toward more biologically inspired methods of oligonucleotide construction. Enzymatic synthesis, which leverages the natural catalytic activity of DNA polymerases, allows for the template-independent or template-driven synthesis of DNA sequences with greater fidelity and fewer errors compared to traditional chemical methods. The use of enzymes such as Terminal Deoxynucleotidyl Transferase (TdT), which adds nucleotides to the 3' end of a DNA strand without the need for a template, provides unique capabilities for labeling, random sequence generation, and oligonucleotide tailing. These enzymatic methods also hold significant potential for the synthesis of longer DNA sequences with enhanced accuracy, a key limitation of chemical methods. As enzymatic synthesis technologies continue to evolve, they are expected to complement or even surpass chemical synthesis in many applications, especially those requiring long, complex, or highly modified DNA sequences.
Another pivotal advancement in synthetic biology is the ability to construct entire genes or even genomes through the assembly of overlapping oligonucleotides. This method, which relies on designing short oligonucleotides with overlapping regions, allows for the stepwise assembly of larger DNA constructs through techniques such as Gibson Assembly, Golden Gate Assembly, and Sequence and Ligation Independent Cloning (SLIC). These methods enable the seamless construction of long, complex DNA sequences with high precision, facilitating the development of novel genetic pathways, synthetic organisms, and engineered biological systems. For example, Gibson Assembly, which uses an exonuclease to create single-stranded overhangs that allow for the precise joining of DNA fragments, has become a cornerstone in the construction of synthetic genes and metabolic pathways. These gene assembly techniques have empowered researchers to design and synthesize entirely new biological constructs, driving forward the fields of synthetic biology, metabolic engineering, and biotechnology.
The future of oligonucleotide synthesis will likely be shaped by continued advancements in both chemical and enzymatic synthesis technologies. Innovations in phosphoramidite chemistry, such as the development of more efficient coupling agents, improved protecting group chemistries, and enhanced automation platforms, will further extend the capabilities of this robust synthesis method, enabling the production of even longer and more complex sequences with fewer synthesis errors. Similarly, ongoing improvements in microarray-based synthesis will allow for the production of longer oligonucleotides at even higher throughput, expanding its utility in high-throughput genomics and synthetic biology applications.
In addition, the integration of next-generation enzymatic synthesis methods with high-fidelity polymerases and the incorporation of modified nucleotides will likely open up new avenues for the creation of highly complex, functionalized oligonucleotides. This will be particularly valuable for applications in therapeutics, where modified oligonucleotides such as antisense oligonucleotides (ASOs), small interfering RNAs (siRNAs), and aptamers are being developed to target specific genetic sequences and modulate gene expression in a highly targeted manner.
Furthermore, as synthetic biology and genome engineering continue to evolve, the ability to design and synthesize entire genetic circuits or even entire genomes will become increasingly important. Techniques for gene synthesis via the assembly of overlapping oligonucleotides will continue to be refined, allowing for the efficient and error-free construction of large, complex genetic constructs. These advances will enable the creation of synthetic organisms with tailored metabolic pathways, novel genetic networks, and engineered traits for applications in biomanufacturing, agriculture, and environmental sustainability.
Overall, oligonucleotide synthesis has become a critical tool for both fundamental research and applied biotechnology, driving innovation in fields as diverse as gene therapy, personalized medicine, synthetic biology, and industrial biotechnology. As these technologies continue to advance, they promise to unlock even greater potential in the understanding and manipulation of nucleic acids, paving the way for new discoveries and therapeutic breakthroughs in the decades to come. The continued refinement of these synthesis methods, coupled with the integration of new technologies such as CRISPR-based genome editing and high-throughput DNA sequencing, will further expand the frontiers of molecular biology, enabling more sophisticated and efficient manipulation of genetic material for a wide range of scientific and clinical applications.


Really enjoyed this